A Novel Therapeutic Agent for Type 2 Diabetes Mellitus: SGLT2 Inhibitor
- Affiliations
-
- 1Department of Internal Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. jypark@amc.seoul.kr
- KMID: 2174102
- DOI: http://doi.org/10.4093/dmj.2014.38.4.261
Abstract
- Type 2 diabetes mellitus (T2DM) is a complex endocrine and metabolic disorder, and a major public health problem that is rapidly increasing in prevalence. Although a wide range of pharmacotherapies for glycemic control is now available, management of T2DM remains complex and challenging. The kidneys contribute immensely to glucose homeostasis by reabsorbing glucose from the glomerular filtrate. Sodium-glucose cotransporter 2 (SGLT2) inhibitors, a new class of antidiabetic agents that inhibit glucose absorption from the kidney independent of insulin, offer a unique opportunity to improve the outcomes of patients with T2DM. In this review, we provide an overview of two globally-approved SGLT2 inhibitors, dapagliflozin and canagliflozin, and discuss their effects and safety. This information will help clinicians to decide whether these drugs will benefit their patients.
MeSH Terms
Figure
Cited by 7 articles
-
Effect of Empagliflozin, a Selective Sodium-Glucose Cotransporter 2 Inhibitor, on Kidney and Peripheral Nerves in Streptozotocin-Induced Diabetic Rats
Kyung Ae Lee, Heung Yong Jin, Na Young Lee, Yu Ji Kim, Tae Sun Park
Diabetes Metab J. 2018;42(4):338-342. doi: 10.4093/dmj.2017.0095.Cardiovascular Safety of Sodium Glucose Cotransporter 2 Inhibitors as Add-on to Metformin Monotherapy in Patients with Type 2 Diabetes Mellitus
Ja Young Jeon, Kyoung Hwa Ha, Dae Jung Kim
Diabetes Metab J. 2021;45(4):505-514. doi: 10.4093/dmj.2020.0057.Ipragliflozin, an SGLT2 Inhibitor, Ameliorates High-Fat Diet-Induced Metabolic Changes by Upregulating Energy Expenditure through Activation of the AMPK/ SIRT1 Pathway
Ji-Yeon Lee, Minyoung Lee, Ji Young Lee, Jaehyun Bae, Eugene Shin, Yong-ho Lee, Byung-Wan Lee, Eun Seok Kang, Bong-Soo Cha
Diabetes Metab J. 2021;45(6):921-932. doi: 10.4093/dmj.2020.0187.Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors
Ji-Yeon Lee, Yongin Cho, Minyoung Lee, You Jin Kim, Yong-ho Lee, Byung-Wan Lee, Bong-Soo Cha, Eun Seok Kang
Diabetes Metab J. 2019;43(2):158-173. doi: 10.4093/dmj.2018.0057.A Lower Baseline Urinary Glucose Excretion Predicts a Better Response to the Sodium Glucose Cotransporter 2 Inhibitor
You-Cheol Hwang, Jae Hyeon Kim, Byung-Wan Lee, Woo Je Lee
Diabetes Metab J. 2019;43(6):898-905. doi: 10.4093/dmj.2018.0257.Effect of Dapagliflozin on Alanine Aminotransferase Improvement in Type 2 Diabetes Mellitus with Non-alcoholic Fatty Liver Disease
Dug-Hyun Choi, Chan-Hee Jung, Ji-Oh Mok, Chul-Hee Kim, Sung-Koo Kang, Bo-Yeon Kim
Endocrinol Metab. 2018;33(3):387-394. doi: 10.3803/EnM.2018.33.3.387.Efficacy and Safety of Evogliptin Add-on Therapy to Dapagliflozin/Metformin Combinations in Patients with Poorly Controlled Type 2 Diabetes Mellitus: A 24-Week Multicenter Randomized Placebo-Controlled Parallel-Design Phase-3 Trial with a 28-Week Extension
Jun Sung Moon, Il Rae Park, Hae Jin Kim, Choon Hee Chung, Kyu Chang Won, Kyung Ah Han, Cheol-Young Park, Jong Chul Won, Dong Jun Kim, Gwan Pyo Koh, Eun Sook Kim, Jae Myung Yu, Eun-Gyoung Hong, Chang Beom Lee, Kun-Ho Yoon
Diabetes Metab J. 2023;47(6):808-817. doi: 10.4093/dmj.2022.0387.
Reference
-
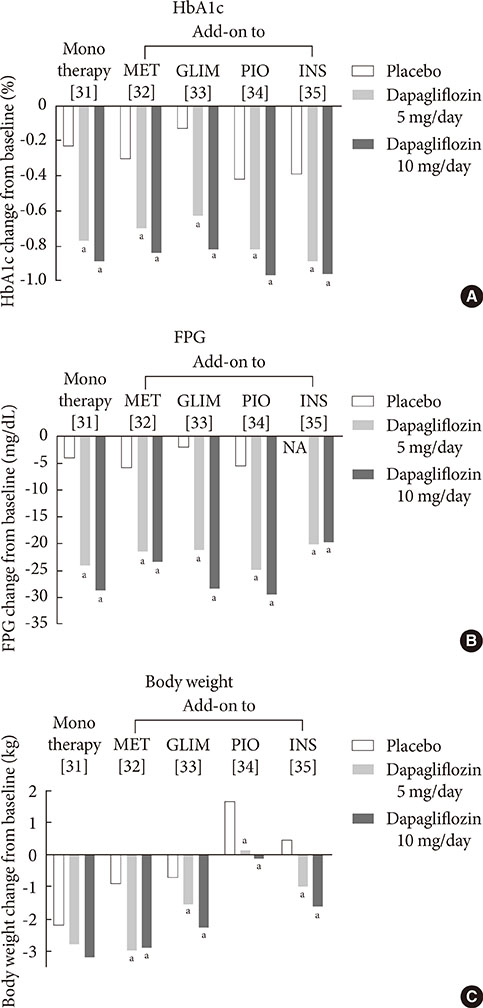
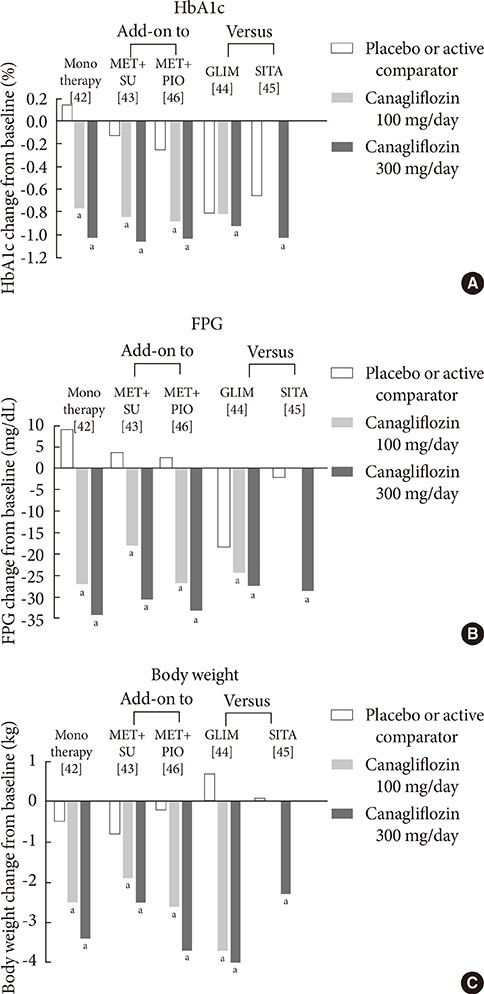
1. Guariguata L. By the numbers: new estimates from the IDF Diabetes Atlas Update for 2012. Diabetes Res Clin Pract. 2012; 98:524–525.2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998; 352:837–853.3. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008; 359:1577–1589.4. Inzucchi SE. Oral antihyperglycemic therapy for type 2 diabetes: scientific review. JAMA. 2002; 287:360–372.5. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. VADT Investigators. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009; 360:129–139.6. Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, Goff DC Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008; 358:2545–2559.7. Chao EC, Henry RR. SGLT2 inhibition: a novel strategy for diabetes treatment. Nat Rev Drug Discov. 2010; 9:551–559.8. Hasan FM, Alsahli M, Gerich JE. SGLT2 inhibitors in the treatment of type 2 diabetes. Diabetes Res Clin Pract. 2014; 104:297–322.9. Raskin P. Sodium-glucose cotransporter inhibition: therapeutic potential for the treatment of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2013; 29:347–356.10. Mather A, Pollock C. Glucose handling by the kidney. Kidney Int Suppl. 2011; (120):S1–S6.11. Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010; 27:136–142.12. Abdul-Ghani MA, Norton L, Defronzo RA. Role of sodium-glucose cotransporter 2 (SGLT 2) inhibitors in the treatment of type 2 diabetes. Endocr Rev. 2011; 32:515–531.13. Wright EM, Hirayama BA, Loo DF. Active sugar transport in health and disease. J Intern Med. 2007; 261:32–43.14. Bays H. From victim to ally: the kidney as an emerging target for the treatment of diabetes mellitus. Curr Med Res Opin. 2009; 25:671–681.15. Gerich JE, Meyer C, Woerle HJ, Stumvoll M. Renal gluconeogenesis: its importance in human glucose homeostasis. Diabetes Care. 2001; 24:382–391.16. Rahmoune H, Thompson PW, Ward JM, Smith CD, Hong G, Brown J. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes. 2005; 54:3427–3434.17. Kamran M, Peterson RG, Dominguez JH. Overexpression of GLUT2 gene in renal proximal tubules of diabetic Zucker rats. J Am Soc Nephrol. 1997; 8:943–948.18. Mogensen CE. Maximum tubular reabsorption capacity for glucose and renal hemodynamcis during rapid hypertonic glucose infusion in normal and diabetic subjects. Scand J Clin Lab Invest. 1971; 28:101–109.19. Freitas HS, Anhe GF, Melo KF, Okamoto MM, Oliveira-Souza M, Bordin S, Machado UF. Na(+) -glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology. 2008; 149:717–724.20. Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009; 58:773–795.21. DeFronzo RA, Davidson JA, Del Prato S. The role of the kidneys in glucose homeostasis: a new path towards normalizing glycaemia. Diabetes Obes Metab. 2012; 14:5–14.22. Ehrenkranz JR, Lewis NG, Kahn CR, Roth J. Phlorizin: a review. Diabetes Metab Res Rev. 2005; 21:31–38.23. Alvarado F, Crane RK. Phlorizin as a competitive inhibitor of the active transport of sugars by hamster small intestine, in vitro. Biochim Biophys Acta. 1962; 56:170–172.24. Rossetti L, Smith D, Shulman GI, Papachristou D, DeFronzo RA. Correction of hyperglycemia with phlorizin normalizes tissue sensitivity to insulin in diabetic rats. J Clin Invest. 1987; 79:1510–1515.25. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011; 91:733–794.26. Thorens B, Mueckler M. Glucose transporters in the 21st Century. Am J Physiol Endocrinol Metab. 2010; 298:E141–E145.27. Elsas LJ, Rosenberg LE. Familial renal glycosuria: a genetic reappraisal of hexose transport by kidney and intestine. J Clin Invest. 1969; 48:1845–1854.28. Plosker GL. Dapagliflozin: a review of its use in type 2 diabetes mellitus. Drugs. 2012; 72:2289–2312.29. European Medicines Agency. European Medicines Agency authorizing the use of dapagliflozin in November 2012. updated 2014 Jul 16. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002322/human_med_001546.jsp&mid=WC0b01ac058001d124.30. U.S. Food and Drug Administration. FDA approves Farxiga to treat type 2 diabetes. updated 2014 Jan 9. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm380829.htm.31. Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010; 33:2217–2224.32. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet. 2010; 375:2223–2233.33. Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24-week, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2011; 13:928–938.34. Rosenstock J, Vico M, Wei L, Salsali A, List JF. Effects of dapagliflozin, an SGLT2 inhibitor, on HbA(1c), body weight, and hypoglycemia risk in patients with type 2 diabetes inadequately controlled on pioglitazone monotherapy. Diabetes Care. 2012; 35:1473–1478.35. Wilding JP, Woo V, Soler NG, Pahor A, Sugg J, Rohwedder K, Parikh S. Dapagliflozin 006 Study Group. Long-term efficacy of dapagliflozin in patients with type 2 diabetes mellitus receiving high doses of insulin: a randomized trial. Ann Intern Med. 2012; 156:405–415.36. Wilding JP, Woo V, Rohwedder K, Sugg J, Parikh S. for the Dapagliflozin 006 Study Group. Dapagliflozin in patients with type 2 diabetes receiving high doses of insulin: efficacy and safety over 2 years. Diabetes Obes Metab. 2013; 98:524–525. Epub 2013 Aug 1. DOI: http://dx.doi.org/10.1111/dom.12187.37. Bolinder J, Ljunggren O, Kullberg J, Johansson L, Wilding J, Langkilde AM, Sugg J, Parikh S. Effects of dapagliflozin on body weight, total fat mass, and regional adipose tissue distribution in patients with type 2 diabetes mellitus with inadequate glycemic control on metformin. J Clin Endocrinol Metab. 2012; 97:1020–1031.38. Henry RR, Murray AV, Marmolejo MH, Hennicken D, Ptaszynska A, List JF. Dapagliflozin, metformin XR, or both: initial pharmacotherapy for type 2 diabetes, a randomised controlled trial. Int J Clin Pract. 2012; 66:446–456.39. Nauck MA, Del Prato S, Meier JJ, Duran-Garcia S, Rohwedder K, Elze M, Parikh SJ. Dapagliflozin versus glipizide as add-on therapy in patients with type 2 diabetes who have inadequate glycemic control with metformin: a randomized, 52-week, double-blind, active-controlled noninferiority trial. Diabetes Care. 2011; 34:2015–2022.40. U.S. Food and Drug Administration. FDA approves Invokana to treat type 2 diabetes. updated 2013 Mar 29. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm345848.htm.41. European Medicines Agency. European Medicines Agency authorizing the use of canagliflozin in November 2013. updated 2013 Nov 30. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002649/human_med_001707.jsp&mid=WC0b01ac058001d124.42. Stenlof K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013; 15:372–382.43. Wilding JP, Charpentier G, Hollander P, Gonzalez-Galvez G, Mathieu C, Vercruysse F, Usiskin K, Law G, Black S, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013; 67:1267–1282.44. Cefalu WT, Leiter LA, Yoon KH, Arias P, Niskanen L, Xie J, Balis DA, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin versus glimepiride in patients with type 2 diabetes inadequately controlled with metformin (CANTATA-SU): 52 week results from a randomised, double-blind, phase 3 non-inferiority trial. Lancet. 2013; 382:941–950.45. Schernthaner G, Gross JL, Rosenstock J, Guarisco M, Fu M, Yee J, Kawaguchi M, Canovatchel W, Meininger G. Canagliflozin compared with sitagliptin for patients with type 2 diabetes who do not have adequate glycemic control with metformin plus sulfonylurea: a 52-week randomized trial. Diabetes Care. 2013; 36:2508–2515.46. Forst T, Guthrie R, Goldenberg R, Yee J, Vijapurkar U, Meininger G, Stein P. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014; 16:467–477.47. Polidori D, Sha S, Mudaliar S, Ciaraldi TP, Ghosh A, Vaccaro N, Farrell K, Rothenberg P, Henry RR. Canagliflozin lowers postprandial glucose and insulin by delaying intestinal glucose absorption in addition to increasing urinary glucose excretion: results of a randomized, placebo-controlled study. Diabetes Care. 2013; 36:2154–2161.48. Lavalle-Gonzalez FJ, Januszewicz A, Davidson J, Tong C, Qiu R, Canovatchel W, Meininger G. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013; 56:2582–2592.49. Devineni D, Morrow L, Hompesch M, Skee D, Vandebosch A, Murphy J, Ways K, Schwartz S. Canagliflozin improves glycaemic control over 28 days in subjects with type 2 diabetes not optimally controlled on insulin. Diabetes Obes Metab. 2012; 14:539–545.50. Liakos A, Karagiannis T, Athanasiadou E, Sarigianni M, Mainou M, Papatheodorou K, Bekiari E, Tsapas A. Efficacy and safety of empagliflozin for type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metab. Epub 2014 Apr 26. DOI: http://dx.doi.org/10.1111/dom.12307.51. Poole RM, Dungo RT. Ipragliflozin: first global approval. Drugs. 2014; 74:611–617.52. Pafili K, Papanas N. Tofogliflozin: the road goes ever on. Expert Opin Pharmacother. 2014; 15:1197–1201.53. Seino Y, Sasaki T, Fukatsu A, Ubukata M, Sakai S, Samukawa Y. Efficacy and safety of luseogliflozin as monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, phase 3 study. Curr Med Res Opin. 2014.54. Miao Z, Nucci G, Amin N, Sharma R, Mascitti V, Tugnait M, Vaz AD, Callegari E, Kalgutkar AS. Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF-04971729) in healthy male subjects. Drug Metab Dispos. 2013; 41:445–456.55. Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Vulvovaginitis and balanitis in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013; 27:479–484.56. Johnsson KM, Ptaszynska A, Schmitz B, Sugg J, Parikh SJ, List JF. Urinary tract infections in patients with diabetes treated with dapagliflozin. J Diabetes Complications. 2013; 27:473–478.57. Nyirjesy P, Sobel JD, Fung A, Mayer C, Capuano G, Ways K, Usiskin K. Genital mycotic infections with canagliflozin, a sodium glucose co-transporter 2 inhibitor, in patients with type 2 diabetes mellitus: a pooled analysis of clinical studies. Curr Med Res Opin. 2014; 30:1109–1119.58. Nicolle LE, Capuano G, Fung A, Usiskin K. Urinary tract infection in randomized phase III studies of canagliflozin, a sodium glucose co-transporter 2 inhibitor. Postgrad Med. 2014; 126:7–17.59. Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab. 2010; 95:34–42.60. Wilding JP, Norwood P, T'Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin-independent treatment. Diabetes Care. 2009; 32:1656–1662.61. Zhang L, Feng Y, List J, Kasichayanula S, Pfister M. Dapagliflozin treatment in patients with different stages of type 2 diabetes mellitus: effects on glycaemic control and body weight. Diabetes Obes Metab. 2010; 12:510–516.62. Ptaszynska A, Chalamandaris AG, Sugg JE, Johnsson KM, Parikh S, List JL. Effect of dapagliflozin on renal function. Diabetes. 2012; 61:Suppl 1. A283.63. Kohan DE, Fioretto P, Tang W, List JF. Long-term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014; 85:962–971.64. Rosenstock J, Aggarwal N, Polidori D, Zhao Y, Arbit D, Usiskin K, Capuano G, Canovatchel W. Canagliflozin DIA 2001 Study Group. Dose-ranging effects of canagliflozin, a sodium-glucose cotransporter 2 inhibitor, as add-on to metformin in subjects with type 2 diabetes. Diabetes Care. 2012; 35:1232–1238.65. Yale JF, Bakris G, Cariou B, Yue D, David-Neto E, Xi L, Figueroa K, Wajs E, Usiskin K, Meininger G. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013; 15:463–473.66. Janssen Research and Development LCC. Canagliflozin slides presented at the Food and Drug Administration (FDA) Endocrinologic and Metabolic Drugs Advisory Committee (EMDAC) meeting, January 10, 2013. updated 2013 Jan 18. Available from: http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/ucm336233.htm.67. Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013; 125:181–189.68. Burki TK. FDA rejects novel diabetes drug over safety fears. Lancet. 2012; 379:507.69. Ptaszynska A, Johnsson KM, Apanovitch AM. Safety of dapagliflozin in clinical trials for T2DM. Diabetes. 2012; 61:Suppl 1. A258.70. FORXIGA. Summary of product characteristics. Wilmington: Bristol-Myers Squibb/AstraZeneca EEIG;2013.71. AstraZeneca Global. AstraZeneca and Bristol-Myers Squibb receive complete response letter from US Food and Drug Administration for dapagliflozin. updated 2012 Jan 19. Available from: http://www.astrazeneca.com/Media/Press-releases/Article/19012012--AstraZeneca-Bristol-Myers-Squibb-receive-CRL-for-dapagliflozin.72. Ghosh RK, Ghosh SM, Chawla S, Jasdanwala SA. SGLT2 inhibitors: a new emerging therapeutic class in the treatment of type 2 diabetes mellitus. J Clin Pharmacol. 2012; 52:457–463.73. Devenny JJ, Godonis HE, Harvey SJ, Rooney S, Cullen MJ, Pelleymounter MA. Weight loss induced by chronic dapagliflozin treatment is attenuated by compensatory hyperphagia in diet-induced obese (DIO) rats. Obesity (Silver Spring). 2012; 20:1645–1652.74. Misra M. SGLT2 inhibitors: a promising new therapeutic option for treatment of type 2 diabetes mellitus. J Pharm Pharmacol. 2013; 65:317–327.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Non-glycemic Effects of SGLT2 Inhibitor
- Glucose Lowering Effect of SGLT2 Inhibitors: A Review of Clinical Studies
- Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitor
- Case of hyperosmolar hyperglycemic state by a sodium-glucose cotransporter 2 inhibitor
- Predictors of the Therapeutic Efficacy and Consideration of the Best Combination Therapy of Sodium-Glucose Co-transporter 2 Inhibitors