J Gynecol Oncol.
2010 Dec;21(4):248-254. 10.3802/jgo.2010.21.4.248.
Comparison of total plasma lysophosphatidic acid and serum CA-125 as a tumor marker in the diagnosis and follow-up of patients with epithelial ovarian cancer
- Affiliations
-
- 1Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Istanbul, Turkey. bese@ttmail.com
- 2Department of Obstetrics and Gynecology, Cerrahpasa Medical School, Istanbul, Turkey.
- 3Institute of Forensic Sciences, Istanbul University, Istanbul, Turkey.
- KMID: 2173549
- DOI: http://doi.org/10.3802/jgo.2010.21.4.248
Abstract
OBJECTIVE
To evaluate the role of lysophosphatidic acid (LPA) as a tumor marker in diagnosis and follow-up of patients with epithelial ovarian cancer.
METHODS
Eighty-seven epithelial ovarian cancer patients, 74 benign ovarian tumor patients, and 50 healthy women were enrolled in the study. Twenty-nine of 87 epithelial ovarian cancer patients were followed up for 6 cycles of paclitaxel-carboplatin chemotherapy. CA-125 and total plasma LPA levels were measured preoperatively and before each chemotherapy cycle.
RESULTS
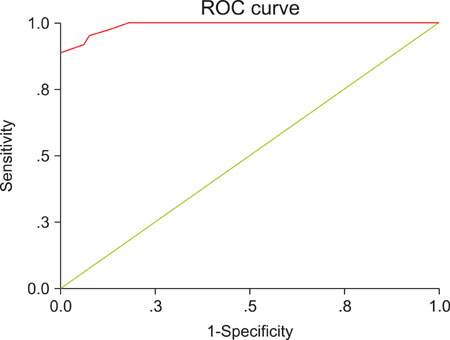
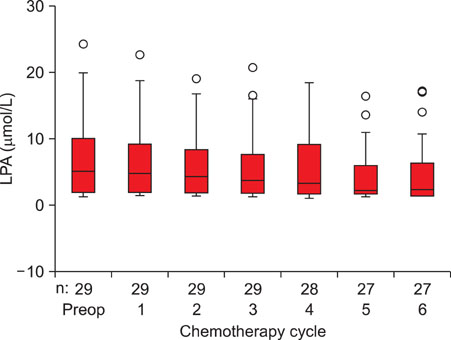
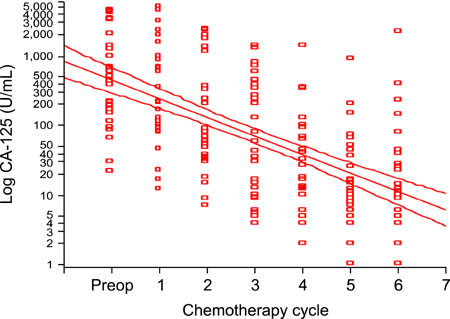
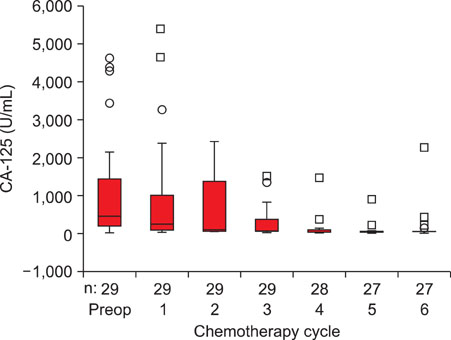
Preoperative total plasma LPA and serum CA-125 levels were significantly higher in patients with epithelial ovarian cancer compared to patients with benign ovarian tumors and healthy women. Cut-off value for LPA was determined as 1.3 micromol/L and sensitivity, specificity, positive predictive value and negative predictive value were 95%, 92%, 95% and 92%, respectively. Mean total plasma LPA level of 29 patients who received chemotherapy was 7.21+/-6.63 micromol/L preoperatively and 6.84+/-6.34 micromol/L, 6.34+/-5.92 micromol/L, 6.14+/-5.79 micromol/L, 5.86+/-5.68 micromol/L, 5.23+/-5.11 micromol/L and 5.21+/-5.32 micromol/L in measurements held just before the 1st, 2nd, 3rd, 4th, 5th and 6th chemotherapy cycles, respectively (ANOVA, p=0.832). Total plasma LPA levels decreased slightly with chemotherapy administration and there was a weak negative correlation (Spearman, rs=-0.151, p=0.034), compared to a significant negative correlation in CA-125 (Spearman, rs=-0.596, p<0.001).
CONCLUSION
LPA is a better biomarker for diagnosis of epithelial ovarian cancer compared to CA-125. However, measurement of total plasma LPA levels during chemotherapy administration have no superiority to the serum CA-125 levels.
Keyword
MeSH Terms
Figure
Reference
-
1. Aoki J, Taira A, Takanezawa Y, Kishi Y, Hama K, Kishimoto T, et al. Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem. 2002. 277:48737–48744.2. Eichholtz T, Jalink K, Fahrenfort I, Moolenaar WH. The bioactive phospholipid lysophosphatidic acid is released from activated platelets. Biochem J. 1993. 291(Pt 3):677–680.3. Xu Y, Shen Z, Wiper DW, Wu M, Morton RE, Elson P, et al. Lysophosphatidic acid as a potential biomarker for ovarian and other gynecologic cancers. JAMA. 1998. 280:719–723.4. Vogt W. Pharamacologically active acidic phospholipids and glycolipids. Biochem Pharmacol. 1963. 12:415–420.5. Xu Y, Gaudette DC, Boynton JD, Frankel A, Fang XJ, Sharma A, et al. Characterization of an ovarian cancer activating factor in ascites from ovarian cancer patients. Clin Cancer Res. 1995. 1:1223–1232.6. Mills GB, May C, McGill M, Roifman CM, Mellors A. A putative new growth factor in ascitic fluid from ovarian cancer patients: identification, characterization, and mechanism of action. Cancer Res. 1988. 48:1066–1071.7. Mills GB, May C, Hill M, Campbell S, Shaw P, Marks A. Ascitic fluid from human ovarian cancer patients contains growth factors necessary for intraperitoneal growth of human ovarian adenocarcinoma cells. J Clin Invest. 1990. 86:851–855.8. Xu Y, Fang XJ, Casey G, Mills GB. Lysophospholipids activate ovarian and breast cancer cells. Biochem J. 1995. 309(Pt 3):933–940.9. Furui T, LaPushin R, Mao M, Khan H, Watt SR, Watt MA, et al. Overexpression of edg-2/vzg-1 induces apoptosis and anoikis in ovarian cancer cells in a lysophosphatidic acid-independent manner. Clin Cancer Res. 1999. 5:4308–4318.10. Schwartz BM, Hong G, Morrison BH, Wu W, Baudhuin LM, Xiao YJ, et al. Lysophospholipids increase interleukin-8 expression in ovarian cancer cells. Gynecol Oncol. 2001. 81:291–300.11. Bast RC Jr, Klug TL, Schaetzl E, Lavin P, Niloff JM, Greber TF, et al. Monitoring human ovarian carcinoma with a combination of CA 125, CA 19-9, and carcinoembryonic antigen. Am J Obstet Gynecol. 1984. 149:553–559.12. Brioschi PA, Irion O, Bischof P, Bader M, Forni M, Krauer F. Serum CA 125 in epithelial ovarian cancer: a longitudinal study. Br J Obstet Gynaecol. 1987. 94:196–201.13. Bast RC Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981. 68:1331–1337.14. Bast RC Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983. 309:883–887.15. Paulsen T, Marth C, Kaern J, Nustad K, Kristensen GB, Trope C. Effects of paclitaxel on CA-125 serum levels in ovarian cancer patients. Gynecol Oncol. 2000. 76:326–330.16. Lavin PT, Knapp RC, Malkasian G, Whitney CW, Berek JC, Bast RC Jr. CA 125 for the monitoring of ovarian carcinoma during primary therapy. Obstet Gynecol. 1987. 69:223–227.17. Dalen A, Favier J, Burges A, Hasholzner U, de Bruijn HW, Dobler-Girdziunaite D, et al. Prognostic significance of CA 125 and TPS levels after 3 chemotherapy courses in ovarian cancer patients. Gynecol Oncol. 2000. 79:444–450.18. Bese T, Nomir SK. The importance of serum insulin-like growth factor-I level determination in the follow-up of patients with epithelial ovarian cancer. Eur J Gynaecol Oncol. 2001. 22:372–376.19. Westermann AM, Havik E, Postma FR, Beijnen JH, Dalesio O, Moolenaar WH, et al. Malignant effusions contain lysophosphatidic acid (LPA)-like activity. Ann Oncol. 1998. 9:437–442.20. Xiao YJ, Schwartz B, Washington M, Kennedy A, Webster K, Belinson J, et al. Electrospray ionization mass spectrometry analysis of lysophospholipids in human ascitic fluids: comparison of the lysophospholipid contents in malignant vs non-malignant ascitic fluids. Anal Biochem. 2001. 290:302–313.21. Baker DL, Morrison P, Miller B, Riely CA, Tolley B, Westermann AM, et al. Plasma lysophosphatidic acid concentration and ovarian cancer. JAMA. 2002. 287:3081–3082.22. Pozlep B, Meleh M, Kobal B, Verdenik I, Osredkar J, Kralj LZ, et al. Use of lysophosphatidic acid in the management of benign and malignant ovarian tumors. Eur J Gynaecol Oncol. 2007. 28:394–399.23. Sedláková I, Vávrová J, Tosner J, Hanousek L. Lysophosphatidic acid: an ovarian cancer marker. Eur J Gynaecol Oncol. 2008. 29:511–514.24. Yu S, Murph MM, Lu Y, Liu S, Hall HS, Liu J, et al. Lysophosphatidic acid receptors determine tumorigenicity and aggressiveness of ovarian cancer cells. J Natl Cancer Inst. 2008. 100:1630–1642.25. Fang X, Gaudette D, Furui T, Mao M, Estrella V, Eder A, et al. Lysophospholipid growth factors in the initiation, progression, metastases, and management of ovarian cancer. Ann N Y Acad Sci. 2000. 905:188–208.26. Fang X, Schummer M, Mao M, Yu S, Tabassam FH, Swaby R, et al. Lysophosphatidic acid is a bioactive mediator in ovarian cancer. Biochim Biophys Acta. 2002. 1582:257–264.27. Goetzl EJ, Dolezalova H, Kong Y, Hu YL, Jaffe RB, Kalli KR, et al. Distinctive expression and functions of the type 4 endothelial differentiation gene-encoded G protein-coupled receptor for lysophosphatidic acid in ovarian cancer. Cancer Res. 1999. 59:5370–5375.28. Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res. 2000. 6:2482–2491.29. Luquain C, Singh A, Wang L, Natarajan V, Morris AJ. Role of phospholipase D in agonist-stimulated lysophosphatidic acid synthesis by ovarian cancer cells. J Lipid Res. 2003. 44:1963–1975.30. Fang X, Yu S, Bast RC, Liu S, Xu HJ, Hu SX, et al. Mechanisms for lysophosphatidic acid-induced cytokine production in ovarian cancer cells. J Biol Chem. 2004. 279:9653–9661.31. Hu YL, Tee MK, Goetzl EJ, Auersperg N, Mills GB, Ferrara N, et al. Lysophosphatidic acid induction of vascular endothelial growth factor expression in human ovarian cancer cells. J Natl Cancer Inst. 2001. 93:762–768.32. Pustilnik TB, Estrella V, Wiener JR, Mao M, Eder A, Watt MA, et al. Lysophosphatidic acid induces urokinase secretion by ovarian cancer cells. Clin Cancer Res. 1999. 5:3704–3710.33. Symowicz J, Adley BP, Woo MM, Auersperg N, Hudson LG, Stack MS. Cyclooxygenase-2 functions as a downstream mediator of lysophosphatidic acid to promote aggressive behavior in ovarian carcinoma cells. Cancer Res. 2005. 65:2234–2242.34. Ren J, Xiao YJ, Singh LS, Zhao X, Zhao Z, Feng L, et al. Lysophosphatidic acid is constitutively produced by human peritoneal mesothelial cells and enhances adhesion, migration, and invasion of ovarian cancer cells. Cancer Res. 2006. 66:3006–3014.35. Tanyi JL, Morris AJ, Wolf JK, Fang X, Hasegawa Y, Lapushin R, et al. The human lipid phosphate phosphatase-3 decreases the growth, survival, and tumorigenesis of ovarian cancer cells: validation of the lysophosphatidic acid signaling cascade as a target for therapy in ovarian cancer. Cancer Res. 2003. 63:1073–1082.36. Baker DL, Fujiwara Y, Pigg KR, Tsukahara R, Kobayashi S, Murofushi H, et al. Carba analogs of cyclic phosphatidic acid are selective inhibitors of autotaxin and cancer cell invasion and metastasis. J Biol Chem. 2006. 281:22786–22793.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The clinical value of serum TPS and CA 125 in the diagnosis of epithelial ovarian cancer
- Diagnostic Efficiency of Peritoneal Fluid and Serum Lactate Dehydrogenase(LDH) in Ovarian Cancer Patients
- Extremely elevated serum CA 125 in a borderline tumor of the ovary: A case report
- Diagnostic Significance of Serum Tumor Markers in Paitents with Ovarian Tumors
- Clinical Relevance of the CA 125 Assay in Monitoring of Epithelial Ovarian Cancer Patients