J Gynecol Oncol.
2008 Dec;19(4):275-278. 10.3802/jgo.2008.19.4.275.
An ovarian mucinous cystadenocarcinoma arising from mature cystic teratoma with para-aortic lymph node metastasis: a case report
- Affiliations
-
- 1Department of Obstetrics and Gynecology, College of Medicine, Inha University, Incheon, Korea. p0928@inha.ac.kr
- 2Department of Pathology, College of Medicine, Inha University, Incheon, Korea.
- KMID: 2173420
- DOI: http://doi.org/10.3802/jgo.2008.19.4.275
Abstract
- Malignant transformation of a mature cystic teratoma (MCT) is an uncommon complication. The most common form of malignant transformation of a MCT is squamous cell carcinoma, representing 75% of malignant transformations. The frequency of malignant transformation of MCT to adenocarcinoma is just 6.8%. To the best of our knowledge, no case of para-aortic lymph node metastasis in mucinous adenocarcinoma arising from MCT has been reported before. The prognosis of malignant transformation of the MCT is very poor. Here, we report an unusual case of a 41-year-old woman with mucinous adenocarcinoma arising from MCT with para-aortic lymph node metastasis.
MeSH Terms
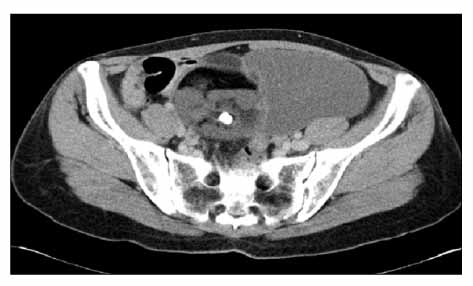
Figure
Reference
-
1. Griffiths D, Wass J, Look K, Sutton G. Malignant degeneration of a mature cystic teratoma five decades after discovery. Gynecol Oncol. 1995. 59:427–429.2. Rose PG, Tak WK, Reale FR. Squamous cell carcinoma arising in a mature cystic teratoma with metastasis to the paraaortic nodes. Gynecol Oncol. 1993. 50:131–133.3. Krumerman MS, Chung A. Squamous carcinoma arising in benign cystic teratoma of the ovary: A report of four cases and review of the literature. Cancer. 1977. 39:1237–1242.4. Curling OM, Potsides PN, Hundson CN. Malignant change in benign cystic teratoma of the ovary. Br J Obstet Gynaecol. 1979. 86:399–402.5. Gordon A, Rosenshein N, Parmley T, Bhagavan B. Benign cystic teratomas in postmenopausal women. Am J Obstet Gynecol. 1980. 138:1120–1123.6. Lee KR, Scully RE. Mucinous tumors of the ovary: A clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with "pseudomyxoma peritonei". Am J Surg Pathol. 2000. 24:1447–1464.7. McKenna JC, Soslow RA, Longacre TA. Mucinous neoplasms arising in mature cystic teratomas: A clinicopathologic study of ovarian and sacrococcygeal tumors. Mod Pathol. 2004. 17:206A.8. Tang P, Soukkary S, Kahn E. Mature cystic teratoma of the ovary associated with complete colonic wall and mucinous cystadenoma. Ann Clin Lab Sci. 2003. 33:465–470.9. Hunter V, Barnhill D, Jadwin D, Crooks L. Ovarian mucinous cystadenocarcinoma of low malignant potential associated with a mature cystic teratoma. Gynecol Oncol. 1988. 29:250–254.10. Fishman A, Edelstein E, Altaras M, Beyth Y, Bernheim J. Adenocarcinoma arising from the gastrointestinal epithelium in benign cystic teratoma of the ovary. Gynecol Oncol. 1998. 70:418–420.11. Fujiwara K, Ginzan S, Silverberg SG. Mature cystic teratomas of the ovary with intestinal wall structures harboring intestinal-type epithelial teratomas. Gynecol Oncol. 1995. 56:97–101.12. Cathro HP, Stoler MH. Expression of cytokeratins 7 and 20 in ovarian neoplasia. Am J Clin Pathol. 2002. 117:944–951.13. Vang R, Gown AM, Barry TS, Wheeler DT, Yemelyanova A, Seidman JD, et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: Analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006. 30:1130–1139.14. Stewart CJ, Tsukamoto T, Cooke B, Leung YC, Hammond IG. Ovarian mucinous tumour arising in mature cystic teratoma and associated with pseudomyxoma peritonei: Report of two cases and comparison with ovarian involvement by low-grade appendiceal mucinous tumour. Pathology. 2006. 38:534–538.15. Fuso L, Amant F, Neven P, Berteloot P, Vergote I. Gemcitabine-carboplatin-paclitaxel combination as first-line therapy in advanced ovarian carcinoma: A single institution phase II study in 24 patients. Int J Gynecol Cancer. 2006. 16:Suppl 1. 60–67.16. Ueda G, Fujita M, Ogawa H, Sawada M, Inoue M, Tanizawa O. Adenocarcinoma in a benign cystic teratoma of the ovary: Report of a case with a long survival period. Gynecol Oncol. 1993. 48:259–263.17. Arora DS, Haldane S. Carcinosarcoma arising in a dermoid cyst of the ovary. J Clin Pathol. 1996. 49:519–521.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Carcinoid Tumor and Low Grade Mucinous Adenocarcinoma Arising in Ovarian Mature Cystic Teratoma
- A Case of Mucinous Cystadenocarcinoma Arising from a Mature Cystic Teratoma in the Right Ovary
- Atypical Proliferating Muinous Tumor arising in a Mature Cystic Teratoma
- A Case of Intestinal Type Mucinous Adenocarcinoma and Strumal Carcinoid Tumor arising in One of Bilateral Mature Cystic Teratoma of the Ovary
- A Case of Adenocarcinoma Arising in Mature Cystic Teratoma of the Ovary