Blood Res.
2015 Sep;50(3):131-139. 10.5045/br.2015.50.3.131.
Hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis: recent advances and controversies
- Affiliations
-
- 1Division of Pediatric Hematology/Oncology, Department of Pediatrics, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea. hojim@amc.seoul.kr
- KMID: 2172755
- DOI: http://doi.org/10.5045/br.2015.50.3.131
Abstract
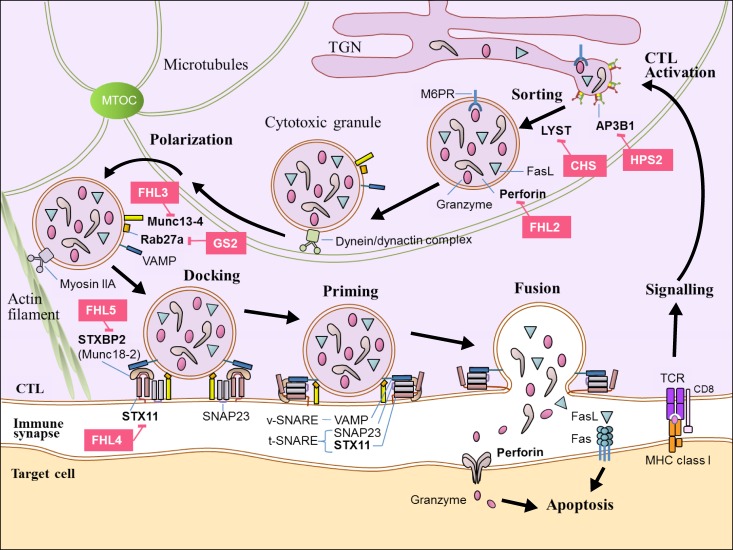
- Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening hyperinflammatory clinical syndrome of uncontrolled immune response which results in hypercytokinemia due to underlying primary or secondary immune defect. A number of genetic defects in transport, processing and function of cytotoxic granules which result in defective granule exocytosis and cytotoxicity of cytotoxic T lymphocytes (CTL) and natural killer (NK) cells have been well identified at the cellular and molecular level. Important advances have been made during the last 20 years in the diagnosis and treatment of HLH. The Histiocyte Society has proposed diagnostic guideline using both clinical and laboratory findings in HLH-2004 protocol, and this has been modified partly in 2009. HLH used to be a fatal disease, but the survival of HLH patients has improved to more than 60% with the use of chemoimmunotherapy combined with hematopoietic cell transplantation (HCT) over the past 2 decades. However, HCT is still the only curative option of treatment for primary HLH and refractory/relapsed HLH after proper chemoimmunotherapy. The outcome of HCT for HLH patients was also improved steadily during last decades, but HCT for HLH still carries significant mortality and morbidity. Moreover, there remain ongoing controversies in various aspects of HCT including indication of HCT, donor selection, timing of HCT, conditioning regimen, and mixed chimerism after HCT. This review summarized the important practical issues which were proven by previous studies on HCT for HLH, and tried to delineate the controversies among them.
MeSH Terms
Figure
Reference
-
1. Sieni E, Cetica V, Hackmann Y, et al. Familial hemophagocytic lymphohistiocytosis: when rare diseases shed light on immune system functioning. Front Immunol. 2014; 5:167. PMID: 24795715.
Article2. Filipovich AH. The expanding spectrum of hemophagocytic lymphohistiocytosis. Curr Opin Allergy Clin Immunol. 2011; 11:512–516. PMID: 21971331.
Article3. Janka GE, Lehmberg K. Hemophagocytic syndromes-an update. Blood Rev. 2014; 28:135–142. PMID: 24792320.4. Janka GE, Lehmberg K. Hemophagocytic lymphohistiocytosis: pathogenesis and treatment. Hematology Am Soc Hematol Educ Program. 2013; 2013:605–611. PMID: 24319239.
Article5. Chandrakasan S, Filipovich AH. Hemophagocytic lymphohistiocytosis: advances in pathophysiology, diagnosis, and treatment. J Pediatr. 2013; 163:1253–1259. PMID: 23953723.
Article6. Bode SF, Lehmberg K, Maul-Pavicic A, et al. Recent advances in the diagnosis and treatment of hemophagocytic lymphohistiocytosis. Arthritis Res Ther. 2012; 14:213. PMID: 22682420.
Article8. Tang YM, Xu XJ. Advances in hemophagocytic lymphohistiocytosis: pathogenesis, early diagnosis/differential diagnosis, and treatment. ScientificWorldJournal. 2011; 11:697–708. PMID: 21442147.
Article9. Gholam C, Grigoriadou S, Gilmour KC, Gaspar HB. Familial haemophagocytic lymphohistiocytosis: advances in the genetic basis, diagnosis and management. Clin Exp Immunol. 2011; 163:271–283. PMID: 21303357.
Article10. Zhao XW, Gazendam RP, Drewniak A, et al. Defects in neutrophil granule mobilization and bactericidal activity in familial hemophagocytic lymphohistiocytosis type 5 (FHL-5) syndrome caused by STXBP2/Munc18-2 mutations. Blood. 2013; 122:109–111. PMID: 23687090.
Article11. Ishii E, Ohga S, Imashuku S, et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int J Hematol. 2007; 86:58–65. PMID: 17675268.
Article12. Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007; 48:124–131. PMID: 16937360.
Article13. Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology Am Soc Hematol Educ Program. 2009; 127–131. PMID: 20008190.
Article14. Janka GE. Familial hemophagocytic lymphohistiocytosis. Eur J Pediatr. 1983; 140:221–230. PMID: 6354720.
Article15. Aricò M, Janka G, Fischer A, et al. FHL Study Group of the Histiocyte Society. Hemophagocytic lymphohistiocytosis. Report of 122 children from the International Registry. Leukemia. 1996; 10:197–203. PMID: 8637226.16. Baker KS, Filipovich AH, Gross TG, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2008; 42:175–180. PMID: 18454181.
Article17. Ohga S, Kudo K, Ishii E, et al. Hematopoietic stem cell transplantation for familial hemophagocytic lymphohistiocytosis and Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in Japan. Pediatr Blood Cancer. 2010; 54:299–306. PMID: 19827139.
Article18. Ouachée-Chardin M, Elie C, de Saint Basile G, et al. Hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis: a single-center report of 48 patients. Pediatrics. 2006; 117:e743–e750. PMID: 16549504.19. Henter JI, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002; 100:2367–2373. PMID: 12239144.
Article20. Naithani R, Asim M, Naqvi A, et al. Increased complications and morbidity in children with hemophagocytic lymphohistiocytosis undergoing hematopoietic stem cell transplantation. Clin Transplant. 2013; 27:248–254. PMID: 23331022.
Article21. Fischer A, Cerf-Bensussan N, Blanche S, et al. Allogeneic bone marrow transplantation for erythrophagocytic lymphohistiocytosis. J Pediatr. 1986; 108:267–270. PMID: 3511206.
Article22. Trottestam H, Horne A, Aricò M, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011; 118:4577–4584. PMID: 21900192.
Article23. Jordan MB, Filipovich AH. Hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis: a journey of a thousand miles begins with a single (big) step. Bone Marrow Transplant. 2008; 42:433–437. PMID: 18679369.
Article24. Baker KS, DeLaat CA, Steinbuch M, et al. Successful correction of hemophagocytic lymphohistiocytosis with related or unrelated bone marrow transplantation. Blood. 1997; 89:3857–3863. PMID: 9160694.
Article25. Cesaro S, Locatelli F, Lanino E, et al. Hematopoietic stem cell transplantation for hemophagocytic lymphohistiocytosis: a retrospective analysis of data from the Italian Association of Pediatric Hematology Oncology (AIEOP). Haematologica. 2008; 93:1694–1701. PMID: 18768529.
Article26. Dürken M, Horstmann M, Bieling P, et al. Improved outcome in haemophagocytic lymphohistiocytosis after bone marrow transplantation from related and unrelated donors: a single-centre experience of 12 patients. Br J Haematol. 1999; 106:1052–1058. PMID: 10520013.
Article27. Horne A, Janka G, Maarten Egeler R, et al. Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis. Br J Haematol. 2005; 129:622–630. PMID: 15916685.
Article28. Imashuku S, Hibi S, Todo S, et al. Allogeneic hematopoietic stem cell transplantation for patients with hemophagocytic syndrome (HPS) in Japan. Bone Marrow Transplant. 1999; 23:569–572. PMID: 10217187.
Article29. Cooper N, Rao K, Gilmour K, et al. Stem cell transplantation with reduced-intensity conditioning for hemophagocytic lymphohistiocytosis. Blood. 2006; 107:1233–1236. PMID: 16219800.
Article30. Malinowska I, Machaczka M, Popko K, Siwicka A, Salamonowicz M, Nasiłowska-Adamska B. Hemophagocytic syndrome in children and adults. Arch Immunol Ther Exp (Warsz). 2014; 62:385–394. PMID: 24509696.
Article31. Cooper N, Rao K, Goulden N, Webb D, Amrolia P, Veys P. The use of reduced-intensity stem cell transplantation in haemophagocytic lymphohistiocytosis and Langerhans cell histiocytosis. Bone Marrow Transplant. 2008; 42(Suppl 2):S47–S50. PMID: 18978744.
Article32. Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL. How I treat hemophagocytic lymphohistiocytosis. Blood. 2011; 118:4041–4052. PMID: 21828139.
Article33. Imashuku S. Clinical features and treatment strategies of Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis. Crit Rev Oncol Hematol. 2002; 44:259–272. PMID: 12467966.
Article34. Imashuku S, Tabata Y, Teramura T, Hibi S. Treatment strategies for Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis (EBV-HLH). Leuk Lymphoma. 2000; 39:37–49. PMID: 10975382.
Article35. Schuster F, Stachel DK, Schmid I, et al. Griscelli syndrome: report of the first peripheral blood stem cell transplant and the role of mutations in the RAB27A gene as an indication for BMT. Bone Marrow Transplant. 2001; 28:409–412. PMID: 11571516.
Article36. Amayiri N, Al-Zaben A, Ghatasheh L, Frangoul H, Hussein AA. Hematopoietic stem cell transplantation for children with primary immunodeficiency diseases: single center experience in Jordan. Pediatr Transplant. 2013; 17:394–402. PMID: 23692601.
Article37. Yoon HS, Im HJ, Moon HN, et al. The outcome of hematopoietic stem cell transplantation in Korean children with hemophagocytic lymphohistiocytosis. Pediatr Transplant. 2010; 14:735–740. PMID: 20113424.
Article38. Caselli D, Aricò M. The role of BMT in childhood histiocytoses. Bone Marrow Transplant. 2008; 41(Suppl 2):S8–S13. PMID: 18545250.
Article39. Mahlaoui N, Ouachée-Chardin M, de Saint Basile G, et al. Immunotherapy of familial hemophagocytic lymphohistiocytosis with antithymocyte globulins: a single-center retrospective report of 38 patients. Pediatrics. 2007; 120:e622–e628. PMID: 17698967.
Article40. Sparber-Sauer M, Hönig M, Schulz AS, et al. Patients with early relapse of primary hemophagocytic syndromes or with persistent CNS involvement may benefit from immediate hematopoietic stem cell transplantation. Bone Marrow Transplant. 2009; 44:333–338. PMID: 19252534.
Article41. Marsh RA, Rao K, Satwani P, et al. Allogeneic hematopoietic cell transplantation for XIAP deficiency: an international survey reveals poor outcomes. Blood. 2013; 121:877–883. PMID: 23131490.
Article42. Satwani P, Cooper N, Rao K, Veys P, Amrolia P. Reduced intensity conditioning and allogeneic stem cell transplantation in childhood malignant and nonmalignant diseases. Bone Marrow Transplant. 2008; 41:173–182. PMID: 18037944.
Article43. Marsh RA, Vaughn G, Kim MO, et al. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010; 116:5824–5831. PMID: 20855862.
Article44. Marsh RA, Kim MO, Liu C, et al. An intermediate alemtuzumab schedule reduces the incidence of mixed chimerism following reduced-intensity conditioning hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Biol Blood Marrow Transplant. 2013; 19:1625–1631. PMID: 24035782.
Article45. Marsh RA, Jordan MB, Filipovich AH. Reduced-intensity conditioning haematopoietic cell transplantation for haemophagocytic lymphohistiocytosis: an important step forward. Br J Haematol. 2011; 154:556–563. PMID: 21707584.
Article46. Lehmberg K, Albert MH, Beier R, et al. Treosulfan-based conditioning regimen for children and adolescents with hemophagocytic lymphohistiocytosis. Haematologica. 2014; 99:180–184. PMID: 24162790.
Article47. Ohta H, Miyashita E, Hirata I, et al. Hematopoietic stem cell transplantation with reduced intensity conditioning from a family haploidentical donor in an infant with familial hemophagocytic lymphohistocytosis. Int J Hematol. 2011; 94:285–290. PMID: 21863286.
Article48. Sawada A, Ohga S, Ishii E, et al. Feasibility of reduced-intensity conditioning followed by unrelated cord blood transplantation for primary hemophagocytic lymphohistiocytosis: a nationwide retrospective analysis in Japan. Int J Hematol. 2013; 98:223–230. PMID: 23843148.
Article49. Nishi M, Nishimura R, Suzuki N, et al. Reduced-intensity conditioning in unrelated donor cord blood transplantation for familial hemophagocytic lymphohistiocytosis. Am J Hematol. 2012; 87:637–639. PMID: 22488407.
Article50. Méresse V, Hartmann O, Vassal G, et al. Risk factors for hepatic veno-occlusive disease after high-dose busulfan-containing regimens followed by autologous bone marrow transplantation: a study in 136 children. Bone Marrow Transplant. 1992; 10:135–141. PMID: 1525602.51. Filipovich A, McClain K, Grom A. Histiocytic disorders: recent insights into pathophysiology and practical guidelines. Biol Blood Marrow Transplant. 2010; 16(1 Suppl):S82–S89. PMID: 19932759.
Article52. Oshrine BR, Olson TS, Bunin N. Mixed chimerism and graft loss in pediatric recipients of an alemtuzumab-based reduced-intensity conditioning regimen for non-malignant disease. Pediatr Blood Cancer. 2014; 61:1852–1859. PMID: 24939325.
Article53. Terrell CE, Jordan MB. Mixed hematopoietic or T-cell chimerism above a minimal threshold restores perforin-dependent immune regulation in perforin-deficient mice. Blood. 2013; 122:2618–2621. PMID: 23974195.
Article54. Shenoy S, Grossman WJ, DiPersio J, et al. A novel reduced-intensity stem cell transplant regimen for nonmalignant disorders. Bone Marrow Transplant. 2005; 35:345–352. PMID: 15592491.
Article55. Koh KN, Im HJ, Chung NG, et al. Clinical features, genetics, and outcome of pediatric patients with hemophagocytic lymphohistiocytosis in Korea: report of a nationwide survey from Korea Histiocytosis Working Party. Eur J Haematol. 2015; 94:51–59. PMID: 24935083.
Article56. Jackson J, Titman P, Butler S, et al. Cognitive and psychosocial function post hematopoietic stem cell transplantation in children with hemophagocytic lymphohistiocytosis. J Allergy Clin Immunol. 2013; 132:889–895.e1-3. PMID: 23987797.
Article57. Abdelkefi A, Ben Jamil W, Torjman L, et al. Hemophagocytic syndrome after hematopoietic stem cell transplantation: a prospective observational study. Int J Hematol. 2009; 89:368–373. PMID: 19252966.
Article58. Asano T, Kogawa K, Morimoto A, et al. Hemophagocytic lymphohistiocytosis after hematopoietic stem cell transplantation in children: a nationwide survey in Japan. Pediatr Blood Cancer. 2012; 59:110–114. PMID: 22038983.
Article59. Koyama M, Sawada A, Yasui M, Inoue M, Kawa K. Encouraging results of low-dose etoposide in the treatment of early-onset hemophagocytic syndrome following allogeneic hematopoietic stem cell transplantation. Int J Hematol. 2007; 86:466–467. PMID: 18192120.
Article60. Stepensky P, Bartram J, Barth TF, et al. Persistent defective membrane trafficking in epithelial cells of patients with familial hemophagocytic lymphohistiocytosis type 5 due to STXBP2/MUNC18-2 mutations. Pediatr Blood Cancer. 2013; 60:1215–1222. PMID: 23382066.61. Trottestam H, Beutel K, Meeths M, et al. Treatment of the X-linked lymphoproliferative, Griscelli and Chédiak-Higashi syndromes by HLH directed therapy. Pediatr Blood Cancer. 2009; 52:268–272. PMID: 18937330.
Article62. Lozano ML, Rivera J, Sánchez-Guiu I, Vicente V. Towards the targeted management of Chediak-Higashi syndrome. Orphanet J Rare Dis. 2014; 9:132. PMID: 25129365.
Article63. Tezcan I, Sanal O, Ersoy F, et al. Successful bone marrow transplantation in a case of Griscelli disease which presented in accelerated phase with neurological involvement. Bone Marrow Transplant. 1999; 24:931–933. PMID: 10516709.
Article64. Pachlopnik Schmid J, Moshous D, Boddaert N, et al. Hematopoietic stem cell transplantation in Griscelli syndrome type 2: a single-center report on 10 patients. Blood. 2009; 114:211–218. PMID: 19403888.
Article65. Booth C, Gilmour KC, Veys P, et al. X-linked lymphoproliferative disease due to SAP/SH2D1A deficiency: a multicenter study on the manifestations, management and outcome of the disease. Blood. 2011; 117:53–62. PMID: 20926771.
Article66. Marsh RA, Bleesing JJ, Chandrakasan S, Jordan MB, Davies SM, Filipovich AH. Reduced-intensity conditioning hematopoietic cell transplantation is an effective treatment for patients with SLAM-associated protein deficiency/X-linked lymphoproliferative disease type 1. Biol Blood Marrow Transplant. 2014; 20:1641–1645. PMID: 24923536.67. Speckmann C, Lehmberg K, Albert MH, et al. X-linked inhibitor of apoptosis (XIAP) deficiency: the spectrum of presenting manifestations beyond hemophagocytic lymphohistiocytosis. Clin Immunol. 2013; 149:133–141. PMID: 23973892.
Article68. Yang X, Kanegane H, Nishida N, et al. Clinical and genetic characteristics of XIAP deficiency in Japan. J Clin Immunol. 2012; 32:411–420. PMID: 22228567.
Article69. Imashuku S, Hyakuna N, Funabiki T, et al. Low natural killer activity and central nervous system disease as a high-risk prognostic indicator in young patients with hemophagocytic lymphohistiocytosis. Cancer. 2002; 94:3023–3031. PMID: 12115393.
Article70. Shuper A, Attias D, Kornreich L, Zaizov R, Yaniv I. Familial hemophagocytic lymphohistiocytosis: improved neurodevelopmental outcome after bone marrow transplantation. J Pediatr. 1998; 133:126–128. PMID: 9672524.
Article71. Decaminada N, Cappellini M, Mortilla M, et al. Familial hemophagocytic lymphohistiocytosis: clinical and neuroradiological findings and review of the literature. Childs Nerv Syst. 2010; 26:121–127. PMID: 19649640.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treosulfan-Based Conditioning Regimen for Hematopoietic Stem Cell Transplantation in Pediatric Patients with Hemophagocytic Lymphohistiocytosis
- Recent advances in histiocytic disorders
- Hemophagocytic Lymphohistiocytosis in Adults: Overview, Diagnosis, and Treatment
- Hemophagocytic Lymphohistiocytosis
- Hemophagocytic Lymphohistiocytosis Occurring after Induction Chemotherapy in Acute Myelocytic Leukemia