Blood Res.
2016 Mar;51(1):31-36. 10.5045/br.2016.51.1.31.
Humanizing NOD/SCID/IL-2Rγnull (NSG) mice using busulfan and retro-orbital injection of umbilical cord blood-derived CD34+ cells
- Affiliations
-
- 1Department of Pediatrics, Korea Cancer Center Hospital, Seoul, Korea. junahlee@kcch.re.kr
- 2Division of Radiation Effect, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 3Laboratory Animal Facility, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- KMID: 2172742
- DOI: http://doi.org/10.5045/br.2016.51.1.31
Abstract
- BACKGROUND
Humanized mouse models are still under development, and various protocols exist to improve human cell engraftment and function.
METHODS
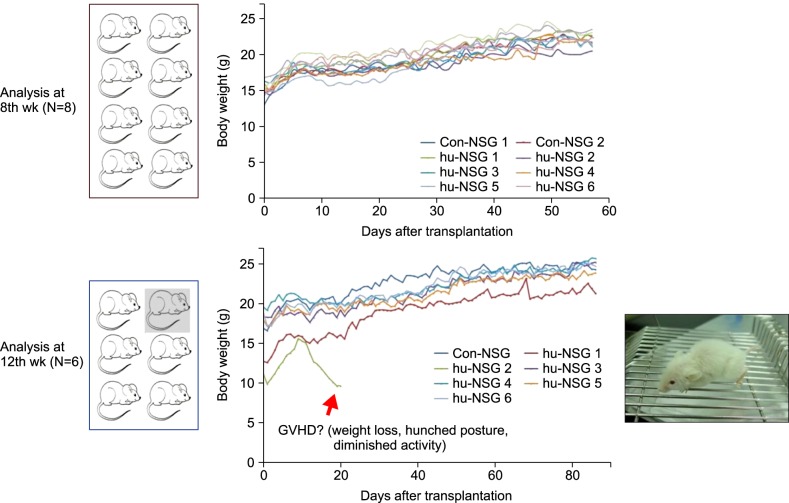
Fourteen NOD/SCID/IL-2Rγnull (NSG) mice (4"’5 wk old) were conditioned with busulfan and injected with human umbilical cord blood (hUCB)-derived CD34+ hematopoietic stem cells (HSC) via retro-orbital sinuses. The bone marrow (BM), spleen, and peripheral blood (PB) were analyzed 8 and 12 weeks after HSC transplantation.
RESULTS
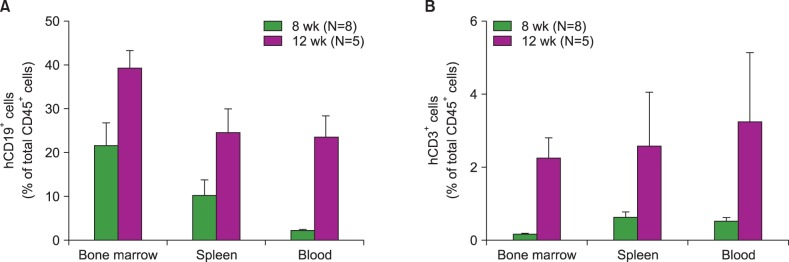
Most of the NSG mice tolerated the regimen well. The percentage of hCD45+ and CD19+ cells rose significantly in a time-dependent manner. The median percentage of hCD45+cells in the BM was 55.5% at week 8, and 67.2% at week 12. The median percentage of hCD45+ cells in the spleen at weeks 8 and 12 was 42% and 51%, respectively. The median percentage of hCD19+ cells in BM at weeks 8 and 12 was 21.5% and 39%, respectively (P=0.04). Similarly, the median percentage of hCD19+ cells in the spleen at weeks 8 and 12 was 10% and 24%, respectively (P=0.04). The percentage of hCD19+ B cells in PB was 23% at week 12. At week 8, hCD3+ T cells were barely detectable, while hCD7+ was detected in the BM and spleen. The percentage of hCD3+ T cells was 2"’3% at week 12 in the BM, spleen, and PB of humanized NSG mice.
CONCLUSION
We adopted a simplified protocol for establishing humanized NSG mice. We observed a higher engraftment rate of human CD45+ cells than earlier studies without any significant toxicity. And human CD45+ cell engraftment at week 8 was comparable to that of week 12.
MeSH Terms
Figure
Reference
-
1. McClure HM. Nonhuman primate models for human disease. Adv Vet Sci Comp Med. 1984; 28:267–304. PMID: 6395673.
Article2. Hu SL. Non-human primate models for AIDS vaccine research. Curr Drug Targets Infect Disord. 2005; 5:193–201. PMID: 15975024.
Article3. Shultz LD, Brehm MA, Garcia-Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol. 2012; 12:786–798. PMID: 23059428.
Article4. Brehm MA, Jouvet N, Greiner DL, Shultz LD. Humanized mice for the study of infectious diseases. Curr Opin Immunol. 2013; 25:428–435. PMID: 23751490.
Article5. King M, Pearson T, Shultz LD, et al. A new Hu-PBL model for the study of human islet alloreactivity based on NOD-scid mice bearing a targeted mutation in the IL-2 receptor gamma chain gene. Clin Immunol. 2008; 126:303–314. PMID: 18096436.
Article6. Harui A, Kiertscher SM, Roth MD. Reconstitution of huPBL-NSG mice with donor-matched dendritic cells enables antigen-specific T-cell activation. J Neuroimmune Pharmacol. 2011; 6:148–157. PMID: 20532647.
Article7. Sugimoto K, Adachi Y, Moriyama K, et al. Induction of the expression of SCF in mouse by lethal irradiation. Growth Factors. 2001; 19:219–231. PMID: 11811778.
Article8. Hayakawa J, Hsieh MM, Uchida N, Phang O, Tisdale JF. Busulfan produces efficient human cell engraftment in NOD/LtSz-Scid IL2Rgamma(null) mice. Stem Cells. 2009; 27:175–182. PMID: 18927475.9. Robert-Richard E, Ged C, Ortet J, et al. Human cell engraftment after busulfan or irradiation conditioning of NOD/SCID mice. Haematologica. 2006; 91:1384. PMID: 17018389.10. Choi B, Chun E, Kim M, et al. Human B cell development and antibody production in humanized NOD/SCID/IL-2Rγ(null) (NSG) mice conditioned by busulfan. J Clin Immunol. 2011; 31:253–264. PMID: 20981478.
Article11. Kim M, Choi B, Kim SY, et al. Co-transplantation of fetal bone tissue facilitates the development and reconstitution in human B cells in humanized NOD/SCID/IL-2Rγnull (NSG) mice. J Clin Immunol. 2011; 31:699–709. PMID: 21544592.
Article12. Chevaleyre J, Duchez P, Rodriguez L, et al. Busulfan administration flexibility increases the applicability of scid repopulating cell assay in NSG mouse model. PLoS One. 2013; 8:e74361. PMID: 24069300.
Article13. Yardeni T, Eckhaus M, Morris HD, Huizing M, Hoogstraten-Miller S. Retro-orbital injections in mice. Lab Anim (NY). 2011; 40:155–160. PMID: 21508954.
Article14. Suckow MA, Danneman P, Brayton C, editors. The laboratory mouse. Boca Raton, FL: CRC Press;2001.15. Price JE, Barth RF, Johnson CW, Staubus AE. Injection of cells and monoclonal antibodies into mice: comparison of tail vein and retroorbital routes. Proc Soc Exp Biol Med. 1984; 177:347–353. PMID: 6091149.
Article16. Steel CD, Stephens AL, Hahto SM, Singletary SJ, Ciavarra RP. Comparison of the lateral tail vein and the retro-orbital venous sinus as routes of intravenous drug delivery in a transgenic mouse model. Lab Anim (NY). 2008; 37:26–32. PMID: 18094699.
Article17. Singh M, Singh P, Gaudray G, et al. An improved protocol for efficient engraftment in NOD/LTSZ-SCIDIL-2Rγnull mice allows HIV replication and development of anti-HIV immune responses. PLoS One. 2012; 7:e38491. PMID: 22675567.
Article18. Lee M, Jeong SY, Ha J, et al. Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun. 2014; 446:983–989. PMID: 24657442.
Article19. Ishikawa F. Modeling normal and malignant human hematopoiesis in vivo through newborn NSG xenotransplantation. Int J Hematol. 2013; 98:634–640. PMID: 24258713.
Article20. Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009; 15:523–536. PMID: 19361744.
Article21. Li Y, Chen Q, Zheng D, et al. Induction of functional human macrophages from bone marrow promonocytes by M-CSF in humanized mice. J Immunol. 2013; 191:3192–3199. PMID: 23935193.
Article22. O'Connell RM, Balazs AB, Rao DS, Kivork C, Yang L, Baltimore D. Lentiviral vector delivery of human interleukin-7 (hIL-7) to human immune system (HIS) mice expands T lymphocyte populations. PLoS One. 2010; 5:e12009. PMID: 20700454.23. Brehm MA, Shultz LD, Luban J, Greiner DL. Overcoming current limitations in humanized mouse research. J Infect Dis. 2013; 208(Suppl 2):S125–S130. PMID: 24151318.
Article24. Drake AC, Chen Q, Chen J. Engineering humanized mice for improved hematopoietic reconstitution. Cell Mol Immunol. 2012; 9:215–224. PMID: 22425741.
Article25. Covassin L, Jangalwe S, Jouvet N, et al. Human immune system development and survival of non-obese diabetic (NOD)-scid IL2rγ(null) (NSG) mice engrafted with human thymus and autologous haematopoietic stem cells. Clin Exp Immunol. 2013; 174:372–388. PMID: 23869841.
Article26. Lavender KJ, Messer RJ, Race B, Hasenkrug KJ. Production of bone marrow, liver, thymus (BLT) humanized mice on the C57BL/6 Rag2(-/-)γc(-/-)CD47(-/-) background. J Immunol Methods. 2014; 407:127–134. PMID: 24769067.
Article27. Choi B, Chun E, Kim M, et al. Human T cell development in the liver of humanized NOD/SCID/IL-2Rγ(null)(NSG) mice generated by intrahepatic injection of CD34(+) human (h) cord blood (CB) cells. Clin Immunol. 2011; 139:321–335. PMID: 21429805.
Article28. van Lent AU, Dontje W, Nagasawa M, et al. IL-7 enhances thymichuman T cell development in "human immune system" Rag2-/- IL-2Rgammac-/- mice without affecting peripheral T cell homeostasis. J Immunol. 2009; 183:7645–7655. PMID: 19923447.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transplantation of human umbilical cord blood CD34⺠cells into the liver of newborn NOD/SCID/IL-2Rγ null (NSG) mice after busulfan conditioning
- Two base pair deletion in IL2 receptor γ gene in NOD/SCID mice induces a highly severe immunodeficiency
- In Vitro Culture of Mast Cells from Human Umbilical Cord Blood Cells
- Effect of Human Parathyroid Hormone on Hematopoietic Progenitor Cells in NOD/SCID Mice Co-Transplanted with Human Cord Blood Mononuclear Cells and Mesenchymal Stem Cells
- Cotransplanted Bone Marrow Derived Mesenchymal Stem Cells (MSC) Enhanced Engraftment of Hematopoietic Stem Cells in a MSC-dose Dependent Manner in NOD/SCID Mice