Blood Res.
2016 Mar;51(1):8-16. 10.5045/br.2016.51.1.8.
Recent advances in haploidentical hematopoietic stem cell transplantation using ex vivo T cell-depleted graft in children and adolescents
- Affiliations
-
- 1Department of Pediatrics, University of Ulsan College of Medicine, Asan Medical Center Children's Hospital, Seoul, Korea. hojim@amc.seoul.kr
- KMID: 2172739
- DOI: http://doi.org/10.5045/br.2016.51.1.8
Abstract
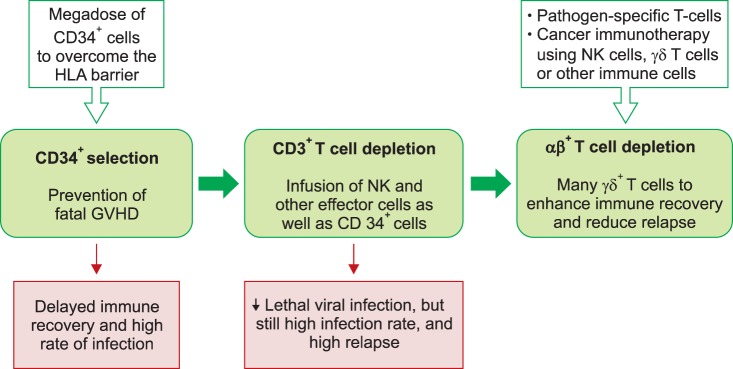
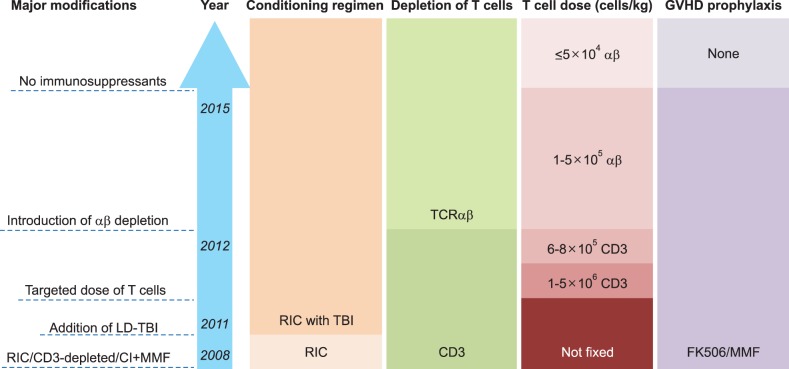
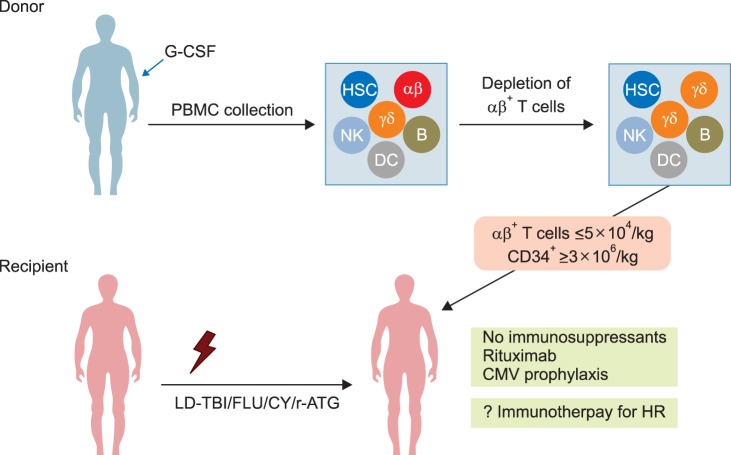
- Allogeneic hematopoietic stem cell transplantation (HSCT) is a curative treatment for children and adolescents with various malignant and non-malignant diseases. While human leukocyte antigen (HLA)-identical sibling donor is the preferred choice, matched unrelated volunteer donor is another realistic option for successful HSCT. Unfortunately, it is not always possible to find a HLA-matched donor for patients requiring HSCT, leading to a considerable number of deaths of patients without undergoing transplantation. Alternatively, allogeneic HSCT from haploidentical family members could provide donors for virtually all patients who need HSCT. Although the early attempts at allogeneic HSCT from haploidentical family donor (HFD) were disappointing, recent advances in the effective ex vivo depletion of T cells or unmanipulated in vivo regulation of T cells, better supportive care, and optimal conditioning regimens have significantly improved the outcomes of haploidentical HSCT. The ex vivo techniques used to remove T cells have evolved from the selection of CD34+ hematopoietic stem cell progenitors to the depletion of CD3+ cells, and more recently to the depletion of αβ+ T cells. The recent emerging evidence for ex vivo T cell-depleted haploidentical HSCT has provided additional therapeutic options for pediatric patients with diseases curable by HSCT but has not found a suitable related or unrelated donor. This review discusses recent advances in haploidentical HSCT, focusing on transplant using ex vivo T cell-depleted grafts. In addition, our experiences with this novel approach for the treatment of pediatric patients with malignant and non-malignant diseases are described.
Keyword
MeSH Terms
Figure
Reference
-
1. Lang P, Teltschik HM, Feuchtinger T, et al. Transplantation of CD3/CD19 depleted allografts from haploidentical family donors in paediatric leukaemia. Br J Haematol. 2014; 165:688–698. PMID: 24588540.2. Im HJ, Koh KN, Suh JK, et al. Refinement of treatment strategies in ex vivo T-cell-depleted haploidentical SCT for pediatric patients. Bone Marrow Transplant. 2015; 50:225–231. PMID: 25310303.
Article3. Airoldi I, Bertaina A, Prigione I, et al. γδT-cell reconstitution after HLA-haploidentical hematopoietic transplantation depleted of TCR-αβ+/CD19+ lymphocytes. Blood. 2015; 125:2349–2358. PMID: 25612623.4. Chang YJ, Huang XJ. Improving the clinical outcome of unmanipulated haploidentical blood and marrow transplantation. Bone Marrow Transplant. 2015; 50(Suppl 2):S21–S23. PMID: 26039202.
Article5. Fuchs EJ. HLA-haploidentical blood or marrow transplantation with high-dose, post-transplantation cyclophosphamide. Bone Marrow Transplant. 2015; 50(Suppl 2):S31–S36. PMID: 26039204.
Article6. Apperley J, Niederwieser D, Huang XJ, et al. Reprint of: haploidentical hematopoietic stem cell transplantation: A global overview comparing Asia, the European Union, and the United States. Biol Blood Marrow Transplant. 2016; 22(Suppl 3):S15–S18. PMID: 26899273.
Article7. Ciurea SO, Bayraktar UD. "No donor"? Consider a haploidentical transplant. Blood Rev. 2015; 29:63–70. PMID: 25307958.
Article8. Or-Geva N, Reisner Y. The evolution of T-cell depletion in haploidentical stem-cell transplantation. Br J Haematol. 2016; 172:667–684. PMID: 26684279.
Article9. Booth C, Lawson S, Veys P. The current role of T cell depletion in paediatric stem cell transplantation. Br J Haematol. 2013; 162:177–190. PMID: 23718232.
Article10. Lang P, Mueller I, Greil J, et al. Retransplantation with stem cells from mismatched related donors after graft rejection in pediatric patients. Blood Cells Mol Dis. 2008; 40:33–39. PMID: 17884640.
Article11. Yoshihara S, Ikegame K, Taniguchi K, et al. Salvage haploidentical transplantation for graft failure using reduced-intensity conditioning. Bone Marrow Transplant. 2012; 47:369–373. PMID: 21478920.
Article12. Park JA, Koh KN, Choi ES, et al. Successful rescue of early graft failure in pediatric patients using T-cell-depleted haploidentical hematopoietic SCT. Bone Marrow Transplant. 2014; 49:270–275. PMID: 24141651.
Article13. Rådestad E, Wikell H, Engström M, et al. Alpha/beta T-cell depleted grafts as an immunological booster to treat graft failure after hematopoietic stem cell transplantation with HLA-matched related and unrelated donors. J Immunol Res. 2014; 2014:578741. PMID: 25371909.
Article14. Powles RL, Morgenstern GR, Kay HE, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983; 1:612–615. PMID: 6131300.
Article15. Beatty PG, Clift RA, Mickelson EM, et al. Marrow transplantation from related donors other than HLA-identical siblings. N Engl J Med. 1985; 313:765–771. PMID: 3897863.
Article16. Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989; 320:197–204. PMID: 2643045.
Article17. Anasetti C, Beatty PG, Storb R, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990; 29:79–91. PMID: 2249952.
Article18. Ash RC, Horowitz MM, Gale RP, et al. Bone marrow transplantation from related donors other than HLA-identical siblings: effect of T cell depletion. Bone Marrow Transplant. 1991; 7:443–452. PMID: 1873591.19. Lang P, Schumm M, Greil J, et al. A comparison between three graft manipulation methods for haploidentical stem cell transplantation in pediatric patients: preliminary results of a pilot study. Klin Padiatr. 2005; 217:334–338. PMID: 16307419.
Article20. Bethge WA, Faul C, Bornhäuser M, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis. 2008; 40:13–19. PMID: 17869547.
Article21. Handgretinger R, Chen X, Pfeiffer M, et al. Feasibility and outcome of reduced-intensity conditioning in haploidentical transplantation. Ann N Y Acad Sci. 2007; 1106:279–289. PMID: 17442774.
Article22. Lang P, Handgretinger R. Haploidentical SCT in children: an update and future perspectives. Bone Marrow Transplant. 2008; 42(Suppl 2):S54–S59. PMID: 18978746.
Article23. Handgretinger R, Lang P. The history and future prospective of haplo-identical stem cell transplantation. Cytotherapy. 2008; 10:443–451. PMID: 18615344.
Article24. Daniele N, Scerpa MC, Caniglia M, et al. Transplantation in the onco-hematology field: focus on the manipulation of αβ and γδ T cells. Pathol Res Pract. 2012; 208:67–73. PMID: 22115749.
Article25. Minculescu L, Sengeløv H. The role of gamma delta T cells in haematopoietic stem cell transplantation. Scand J Immunol. 2015; 81:459–468. PMID: 25753378.
Article26. Gordon PR, Leimig T, Mueller I, et al. A large-scale method for T cell depletion: towards graft engineering of mobilized peripheral blood stem cells. Bone Marrow Transplant. 2002; 30:69–74. PMID: 12132044.
Article27. Barfield RC, Otto M, Houston J, et al. A one-step large-scale method for T- and B-cell depletion of mobilized PBSC for allogeneic transplantation. Cytotherapy. 2004; 6:1–6. PMID: 14985161.
Article28. Handgretinger R, Klingebiel T, Lang P, et al. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001; 27:777–783. PMID: 11477433.29. Lang P, Greil J, Bader P, et al. Long-term outcome after haploidentical stem cell transplantation in children. Blood Cells Mol Dis. 2004; 33:281–287. PMID: 15528145.
Article30. Locatelli F, Pende D, Maccario R, Mingari MC, Moretta A, Moretta L. Haploidentical hemopoietic stem cell transplantation for the treatment of high-risk leukemias: how NK cells make the difference. Clin Immunol. 2009; 133:171–178. PMID: 19481979.
Article31. Bader P, Soerensen J, Jarisch A, et al. Rapid immune recovery and low TRM in haploidentical stem cell transplantation in children and adolescence using CD3/CD19-depleted stem cells. Best Pract Res Clin Haematol. 2011; 24:331–337. PMID: 21925086.
Article32. Palma J, Salas L, Carrión F, et al. Haploidentical stem cell transplantation for children with high-risk leukemia. Pediatr Blood Cancer. 2012; 59:895–901. PMID: 22238059.
Article33. González-Vicent M, Molina B, Andión M, et al. Allogeneic hematopoietic transplantation using haploidentical donor vs. unrelated cord blood donor in pediatric patients: a single-center retrospective study. Eur J Haematol. 2011; 87:46–53. PMID: 21692851.
Article34. Pérez-Martínez A, González-Vicent M, Valentín J, et al. Early evaluation of immune reconstitution following allogeneic CD3/CD19-depleted grafts from alternative donors in childhood acute leukemia. Bone Marrow Transplant. 2012; 47:1419–1427. PMID: 22410752.
Article35. Dufort G, Pisano S, Incoronato A, et al. Feasibility and outcome of haploidentical SCT in pediatric high-risk hematologic malignancies and Fanconi anemia in Uruguay. Bone Marrow Transplant. 2012; 47:663–668. PMID: 21765479.
Article36. Chen X, Hale GA, Barfield R, et al. Rapid immune reconstitution after a reduced-intensity conditioning regimen and a CD3-depleted haploidentical stem cell graft for paediatric refractory haematological malignancies. Br J Haematol. 2006; 135:524–532. PMID: 17010105.
Article37. Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol. 2013; 13:88–100. PMID: 23348415.
Article38. Norell H, Moretta A, Silva-Santos B, Moretta L. At the Bench: Preclinical rationale for exploiting NK cells and γδ T lymphocytes for the treatment of high-risk leukemias. J Leukoc Biol. 2013; 94:1123–1139. PMID: 24108703.
Article39. Hu Y, Cui Q, Luo C, Luo Y, Shi J, Huang H. A promising sword of tomorrow: Human γδ T cell strategies reconcile allo-HSCT complications. Blood Rev. 2015; [Epub ahead of print].
Article40. Lamb LS Jr, Lopez RD. gammadelta T cells: a new frontier for immunotherapy? Biol Blood Marrow Transplant. 2005; 11:161–168. PMID: 15744234.41. Locatelli F, Bauquet A, Palumbo G, Moretta F, Bertaina A. Negative depletion of α/β+ T cells and of CD19+ B lymphocytes: a novel frontier to optimize the effect of innate immunity in HLA-mismatched hematopoietic stem cell transplantation. Immunol Lett. 2013; 155:21–23. PMID: 24091162.
Article42. Handgretinger R. New approaches to graft engineering for haploidentical bone marrow transplantation. Semin Oncol. 2012; 39:664–673. PMID: 23206843.
Article43. Schumm M, Lang P, Bethge W, et al. Depletion of T-cell receptor alpha/beta and CD19 positive cells from apheresis products with the CliniMACS device. Cytotherapy. 2013; 15:1253–1258. PMID: 23993299.
Article44. Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood. 2014; 124:822–826. PMID: 24869942.
Article45. Maschan M, Shelikhova L, Ilushina M, et al. TCR-alpha/beta and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone Marrow Transplant. 2016; [Epub ahead of print].
Article46. Lang P, Feuchtinger T, Teltschik HM, et al. Improved immune recovery after transplantation of TCRαβ/CD19-depleted allografts from haploidentical donors in pediatric patients. Bone Marrow Transplant. 2015; 50(Suppl 2):S6–S10. PMID: 26039210.
Article47. Sarkodee-Adoo C, Sotirescu D, Sensenbrenner L, et al. Thrombotic microangiopathy in blood and marrow transplant patients receiving tacrolimus or cyclosporine A. Transfusion. 2003; 43:78–84. PMID: 12519434.48. Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005; 11:571–575. PMID: 16041306.
Article49. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334:494–500. PMID: 8559202.
Article50. Koh KN, Park M, Kim BE, Im HJ, Seo JJ. Early central nervous system complications after allogeneic hematopoietic stem cell transplantation in children. Korean J Hematol. 2010; 45:164–170. PMID: 21120204.
Article51. Ciceri F, Lupo-Stanghellini MT, Korthof ET. Haploidentical transplantation in patients with acquired aplastic anemia. Bone Marrow Transplant. 2013; 48:183–185. PMID: 23292235.
Article52. Im HJ, Koh KN, Choi ES, et al. Excellent outcome of haploidentical hematopoietic stem cell transplantation in children and adolescents with acquired severe aplastic anemia. Biol Blood Marrow Transplant. 2013; 19:754–759. PMID: 23380343.53. Clay J, Kulasekararaj AG, Potter V, et al. Nonmyeloablative peripheral blood haploidentical stem cell transplantation for refractory severe aplastic anemia. Biol Blood Marrow Transplant. 2014; 20:1711–1716. PMID: 25016195.
Article54. Gao L, Li Y, Zhang Y, et al. Long-term outcome of HLA-haploidentical hematopoietic SCT without in vitro T-cell depletion for adult severe aplastic anemia after modified conditioning and supportive therapy. Bone Marrow Transplant. 2014; 49:519–524. PMID: 24464145.
Article55. Wang Z, Zheng X, Yan H, Li D, Wang H. Good outcome of haploidentical hematopoietic SCT as a salvage therapy in children and adolescents with acquired severe aplastic anemia. Bone Marrow Transplant. 2014; 49:1481–1485. PMID: 25133891.
Article56. Esteves I, Bonfim C, Pasquini R, et al. Haploidentical BMT and post-transplant Cy for severe aplastic anemia: a multicenter retrospective study. Bone Marrow Transplant. 2015; 50:685–689. PMID: 25730184.
Article57. Im HJ, Koh KN, Seo JJ. Haploidentical hematopoietic stem cell transplantation in children and adolescents with acquired severe aplastic anemia. Korean J Pediatr. 2015; 58:199–205. PMID: 26213547.
Article58. Liu L, Wang X, Jin S, et al. Haploidentical hematopoietic stem cell transplantation for nonresponders to immunosuppressive therapy against acquired severe aplastic anemia. Bone Marrow Transplant. 2016; 51:424–427. PMID: 26479978.
Article59. Teschner D, Distler E, Wehler D, et al. Depletion of naive T cells using clinical grade magnetic CD45RA beads: a new approach for GVHD prophylaxis. Bone Marrow Transplant. 2014; 49:138–144. PMID: 23933765.
Article60. Triplett BM, Shook DR, Eldridge P, et al. Rapid memory T-cell reconstitution recapitulating CD45RA-depleted haploidentical transplant graft content in patients with hematologic malignancies. Bone Marrow Transplant. 2015; 50:968–977. PMID: 25665048.
Article61. Bleakley M, Heimfeld S, Loeb KR, et al. Outcomes of acute leukemia patients transplanted with naive T cell-depleted stem cell grafts. J Clin Invest. 2015; 125:2677–2689. PMID: 26053664.
Article62. Feuchtinger T, Opherk K, Bethge WA, et al. Adoptive transfer of pp65-specific T cells for the treatment of chemorefractory cytomegalovirus disease or reactivation after haploidentical and matched unrelated stem cell transplantation. Blood. 2010; 116:4360–4367. PMID: 20625005.
Article63. Feucht J, Joachim L, Lang P, Feuchtinger T. Adoptive T-cell transfer for refractory viral infections with cytomegalovirus, Epstein-Barr virus or adenovirus after allogeneic stem cell transplantation. Klin Padiatr. 2013; 225:164–169. PMID: 23700092.
Article64. Gerdemann U, Katari UL, Papadopoulou A, et al. Safety and clinical efficacy of rapidly-generated trivirus-directed T cells as treatment for adenovirus, EBV, and CMV infections after allogeneic hematopoietic stem cell transplant. Mol Ther. 2013; 21:2113–2121. PMID: 23783429.
Article65. Icheva V, Kayser S, Wolff D, et al. Adoptive transfer of epsteinbarr virus (EBV) nuclear antigen 1-specific t cells as treatment for EBV reactivation and lymphoproliferative disorders after allogeneic stem-cell transplantation. J Clin Oncol. 2013; 31:39–48. PMID: 23169501.
Article66. Wilhelm M, Kunzmann V, Eckstein S, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003; 102:200–206. PMID: 12623838.67. Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res. 2010; 70:10024–10027. PMID: 21159627.68. Fisher JP, Heuijerjans J, Yan M, Gustafsson K, Anderson J. γδ T cells for cancer immunotherapy: A systematic review of clinical trials. Oncoimmunology. 2014; 3:e27572. PMID: 24734216.69. Wilhelm M, Smetak M, Schaefer-Eckart K, et al. Successful adoptive transfer and in vivo expansion of haploidentical γδ T cells. J Transl Med. 2014; 12:45. PMID: 24528541.
Article70. Rubnitz JE, Inaba H, Ribeiro RC, et al. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010; 28:955–959. PMID: 20085940.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Haploidentical hematopoietic stem cell transplantation in children and adolescents with acquired severe aplastic anemia
- Haploidentical Family Donor Transplantation for Pediatric Hematologic Malignancies
- New therapeutic modalities on hematopoietic stem cell transplantation
- Strategies to enhance graft performance in cord blood transplantation
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation