Chonnam Med J.
2013 Apr;49(1):20-26. 10.4068/cmj.2013.49.1.20.
Clinical Usefulness of 18F-FDG PET/CT in the Detection of Early Recurrence in Treated Cervical Cancer Patients with Unexplained Elevation of Serum Tumor Markers
- Affiliations
-
- 1Department of Nuclear Medicine, Chosun University Hospital, Gwangju, Korea.
- 2Kyungpook National University Hospital, Daegu, Korea.
- 3Chonnam National University Hospital, Gwangju, Korea. songhc@jnu.ac.kr
- 4Department of Obstetrics and Gynecology, Chonnam National University Hospital, Gwangju, Korea.
- KMID: 2172172
- DOI: http://doi.org/10.4068/cmj.2013.49.1.20
Abstract
- We investigated the diagnostic value of 18F-fluorodeoxyglucose positron emission tomography/computed tomography (PET/CT) for restaging of treated uterine cervix squamous cell cancer with tumor maker elevation that was not explained by other conventional evaluation. We enrolled 32 cases who underwent PET/CT for the restaging of treated cervical cancer with tumor marker elevation that was not explained by recent conventional evaluation. All enrolled cases had squamous cell carcinoma. Increased tumor markers included squamous cell carcinoma antigen (SCC Ag) and carcinoembryonic antigen (CEA). PET/CT findings were determined by pathologic confirmation or clinical follow-up. We compared PET/CT accuracy and clinical parameters including normalization of tumor markers in both the SCC Ag elevation group and the CEA elevation group. The sensitivity, specificity, positive predictive value, and negative predictive value of PET/CT in detecting recurrence were 100%, 83.3%, 82.4%, and 100%, respectively. Accuracy was significantly different between the SCC Ag elevation group and the CEA elevation group (p=0.0169). PET/CT with SCC Ag elevation was more accurate (100%) than PET/CT with CEA elevation (66.7%). Normalization of tumor markers was observed more often in the SCC Ag elevation group than in the CEA elevation group (p=0.0429). PET/CT showed high negative predictive value and sensitivity in the restaging of cervical cancer with unexplained tumor marker elevation. PET/CT was more accurate in patients with SCC Ag elevation than in those with CEA elevation.
MeSH Terms
-
Antigens, Neoplasm
Carcinoembryonic Antigen
Carcinoma, Squamous Cell
Cervix Uteri
Electrons
Female
Fluorodeoxyglucose F18
Follow-Up Studies
Humans
Neoplasms, Squamous Cell
Positron-Emission Tomography and Computed Tomography
Recurrence
Sensitivity and Specificity
Serpins
Biomarkers, Tumor
Uterine Cervical Neoplasms
Antigens, Neoplasm
Carcinoembryonic Antigen
Fluorodeoxyglucose F18
Serpins
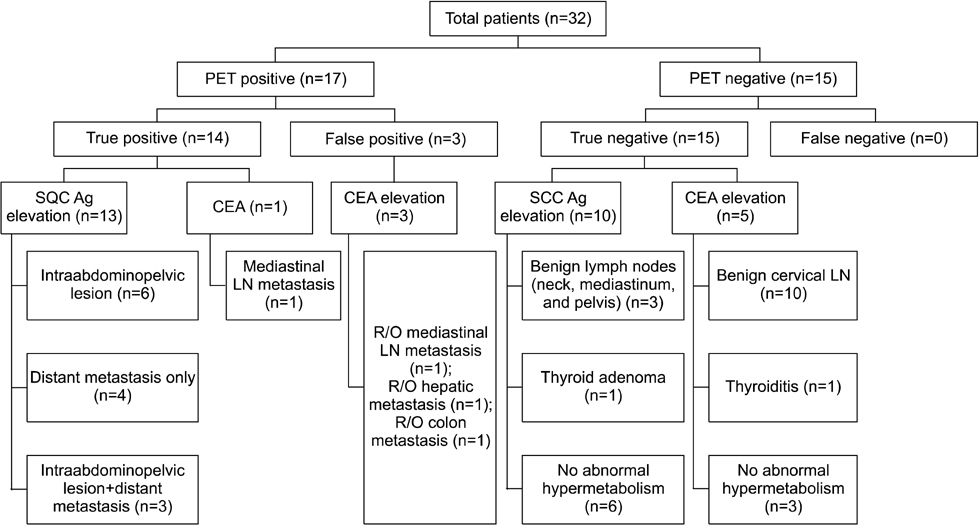
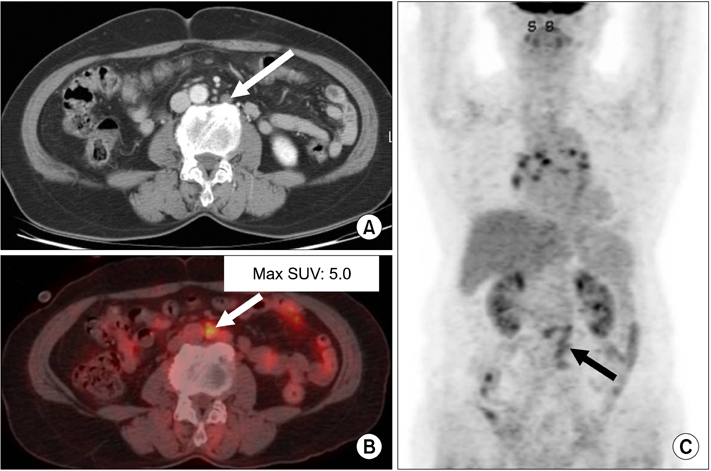
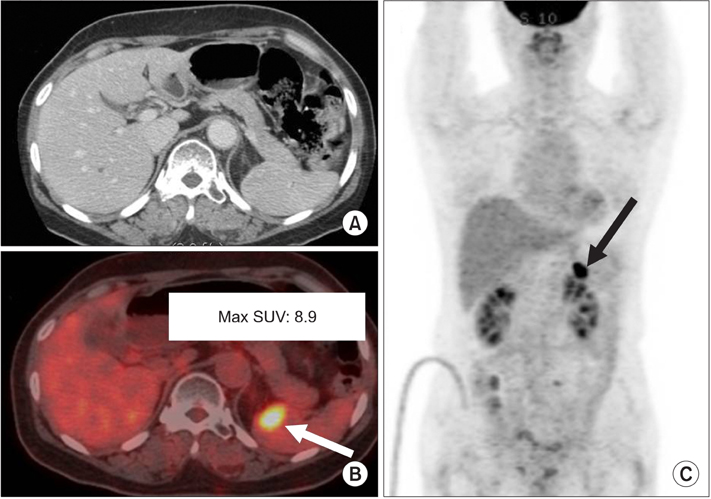
Figure
Reference
-
1. Ellenson LH, Wu TC. Focus on endometrial and cervical cancer. Cancer Cell. 2004. 5:533–538.
Article2. Oh SW, Kim SK. Clinical application of 18F-FDG PET in cervix cancer. Nucl Med Mol Imaging. 2008. 42:suppl 1. 101–109.3. Brady LW, Perez CA, Bedwinek JM. Failure patterns in gynecologic cancer. Int J Radiat Oncol Biol Phys. 1986. 12:549–557.
Article4. Fagundes H, Perez CA, Grigsby PW, Lockett MA. Distant metastases after irradiation alone in carcinoma of the uterine cervix. Int J Radiat Oncol Biol Phys. 1992. 24:197–204.
Article5. Perez CA, Breaux S, Madoc-Jones H, Bedwinek JM, Camel HM, Purdy JA, et al. Radiation therapy alone in the treatment of carcinoma of uterine cervix I Analysis of tumor recurrence. Cancer. 1983. 51:1393–1402.
Article6. Benedet JL, Odicino F, Maisonneuve P, Beller U, Creasman WT, Heintz AP, et al. Carcinoma of the cervix uteri. J Epidemiol Biostat. 2001. 6:7–43.
Article7. Larson DM, Copeland LJ, Stringer CA, Gershenson DM, Malone JM Jr, Edwards CL. Recurrent cervical carcinoma after radical hysterectomy. Gynecol Oncol. 1988. 30:381–387.
Article8. Irvin WP, Rice LW, Berkowitz RS. Advances in the management of endometrial adenocarcinoma. A review. J Reprod Med. 2002. 47:173–189.9. Bodurka-Bevers D, Morris M, Eifel PJ, Levenback C, Bevers MW, Lucas KR, et al. Posttherapy surveillance of women with cervical cancer: an outcomes analysis. Gynecol Oncol. 2000. 78:187–193.
Article10. Sakurai H, Suzuki Y, Nonaka T, Ishikawa H, Shioya M, Kiyohara H, et al. FDG-PET in the detection of recurrence of uterine cervical carcinoma following radiation therapy--tumor volume and FDG uptake value. Gynecol Oncol. 2006. 100:601–607.
Article11. Gadducci A, Tana R, Cosio S, Genazzani AR. The serum assay of tumour markers in the prognostic evaluation, treatment monitoring and follow-up of patients with cervical cancer: a review of the literature. Crit Rev Oncol Hematol. 2008. 66:10–20.
Article12. Chan YM, Ng TY, Ngan HY, Wong LC. Monitoring of serum squamous cell carcinoma antigen levels in invasive cervical cancer: is it cost-effective? Gynecol Oncol. 2002. 84:7–11.
Article13. Lozza L, Merola M, Fontanelli R, Stefanon B, Seregni E, Bombardieri E, et al. Cancer of the uterine cervix: clinical value of squamous cell carcinoma antigen (SCC) measurements. Anticancer Res. 1997. 17:525–529.14. Micke O, Prott FJ, Schäfer U, Tangerding S, Pötter R, Willich N. The impact of squamous cell carcinoma (SCC) antigen in the follow-up after radiotherapy in patients with cervical cancer. Anticancer Res. 2000. 20:5113–5115.15. Chang TC, Law KS, Hong JH, Lai CH, Ng KK, Hsueh S, et al. Positron emission tomography for unexplained elevation of serum squamous cell carcinoma antigen levels during follow-up for patients with cervical malignancies: a phase II study. Cancer. 2004. 101:164–171.
Article16. Chang WC, Hung YC, Lin CC, Shen YY, Kao CH. Usefulness of FDG-PET to detect recurrent cervical cancer based on asymptomatically elevated tumor marker serum levels--a preliminary report. Cancer Invest. 2004. 22:180–184.
Article17. Chung HH, Jo H, Kang WJ, Kim JW, Park NH, Song YS, et al. Clinical impact of integrated PET/CT on the management of suspected cervical cancer recurrence. Gynecol Oncol. 2007. 104:529–534.
Article18. Hu YY, Sun XR, Lin XP, Liang PY, Zhang X, Fan W. Application of 18F-FDG PET/CT in cervical cancer with elevated levels of serum squamous cell carcinoma antigen during the follow-up. Ai Zheng. 2009. 28:994–999.
Article19. Numa F, Takeda O, Nakata M, Nawata S, Tsunaga N, Hirabayashi K, et al. Tumor necrosis factor-alpha stimulates the production of squamous cell carcinoma antigen in normal squamous cells. Tumour Biol. 1996. 17:97–101.
Article20. Cases A, Filella X, Molina R, Ballesta AM, Lopez-Pedret J, Revert L. Tumor markers in chronic renal failure and hemodialysis patients. Nephron. 1991. 57:183–186.
Article21. Tramonti G, Ferdeghini M, Donadio C, Norpoth M, Annichiarico C, Bianchi R, et al. Renal function and serum concentration of five tumor markers (TATI, SCC, CYFRA 21-1, TPA, and TPS) in patients without evidence of neoplasia. Cancer Detect Prev. 2000. 24:86–90.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of 18F-FDG PET/CT in Second Primary Cancer
- The Role of 18F-FDG PET/CT in the Evaluation of Gastric Cancer Recurrence after Curative Gastrectomy
- Opportunities for 2-[18F] Fluoro-2-Deoxy-D-Glucose PET/CT in Cervical-Vaginal Neuroendocrine Carcinoma: Case Series and Literature Review
- Small Animal [18F]FDG PET Imaging for Tumor Model Study
- Accuracy of [ 18F ] FDG PET after Surgery and Radiotherapy in Head and Neck Cancers