Ann Dermatol.
2010 Feb;22(1):9-15. 10.5021/ad.2010.22.1.9.
The Efficacy and Safety of Long-term Oral Cyclosporine Treatment for Patients with Atopic Dermatitis
- Affiliations
-
- 1Department of Dermatology, School of Medicine, Kyung Hee University, Seoul, Korea. crhaw@khmc.or.kr
- KMID: 2172030
- DOI: http://doi.org/10.5021/ad.2010.22.1.9
Abstract
- BACKGROUND
Steroids are used in conventional treatment of atopic dermatitis (AD) and they are very effective for improving the symptoms, but they also have several complications. Many studies have reported that short-term use of cyclosporine (CsA) is effective for severe AD as a substitute for steroid. However, there are very few studies on the long-term use of CsA for AD in the Korean population.
OBJECTIVE
The purpose of this study was to investigate whether long-term CsA therapy is effective and safe for treating AD.
METHODS
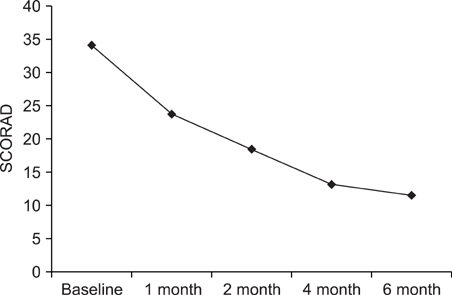
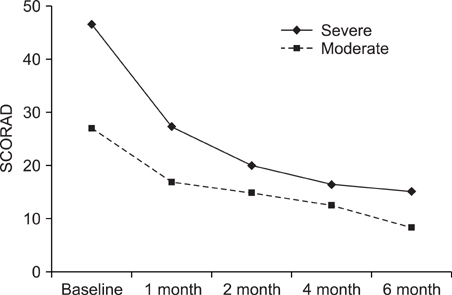
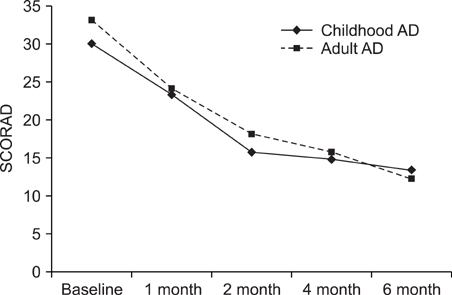
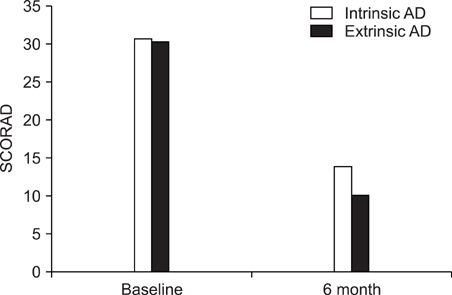
We performed a retrospective study of the patients with AD and who were treated with CsA at Kyung Hee Medical Center between January 2001 and February 2008. Among 147 patients, 61 received CsA treatment for more than 6 months. To evaluate the efficacy of CsA treatment, the objective SCORAD was checked for all 61 patients at every visit. Extensive laboratory tests were performed every two months to assess the safety of treatment. RESULTS: The mean duration of CsA treatment was 13.5+/-8.4 months and the mean initial dose of CsA was 2.7+/-0.9 mg/kg/day. The mean objective SCORAD values significantly decreased from 34.1+/-11.2 at baseline to 11.4+/-10.7 after 6-month of CsA treatment (p<0.05). A significant decline of the SCORAD score was observed starting from 1-month of CsA treatment. The mean duration of remission was 4.5+/-2.9 months. A total of 13 adverse events in 10 patients were recorded during the study period. One patient dropped out due to renal dysfunction. Elevation of peripheral blood pressure was noted in 8 patients. Three patients complained of gastrointestinal troubles, and one patient had hypertrichosis, but the problems of these 4 patients were mild and easily treated.
CONCLUSION
We suggest that long-term, low-dose CsA treatment is safe and effective for patients who suffer from AD.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Risk Factors Affecting Adverse Effects of Cyclosporine A in a Real-World Psoriasis Treatment
Bo Ri Kim, Seungkeol Yang, Eun Jin Doh, Chong Won Choi, Sang Woong Youn
Ann Dermatol. 2018;30(2):143-149. doi: 10.5021/ad.2018.30.2.143.
Reference
-
1. James WD, Berger TG, Elston DM, Odom RB. Andrews' diseases of the skin: clinical dermatology. 2006. 10th ed. Philadelphia: Saunders Elsevier;69–77.2. Borel JF, Feurer C, Gubler HU, Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976. 6:468–475.
Article3. Mueller W, Herrmann B. Cyclosporin A for psoriasis. N Engl J Med. 1979. 301:555.
Article4. Nousari CH, Anhalt GJ. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Immunosuppressive and immunomodulatory drugs. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;2217–2223.5. Lee CS, Koo JYM. Wolverton SE, editor. Cyclosporine. Comprehensive dermatologic drug therapy. 2007. 2nd ed. Indiana: Saunders Elsevier;219–238.6. Hijnen DJ, ten Berge O, Timmer-de Mik L, Bruijnzeel-Koomen CA, de Bruin-Weller MS. Efficacy and safety of long-term treatment with cyclosporin A for atopic dermatitis. J Eur Acad Dermatol Venereol. 2007. 21:85–89.
Article7. Harper JI, Berth-Jones J, Camp RD, Dillon MJ, Finlay AY, Holden CA, et al. Cyclosporin for atopic dermatitis in children. Dermatology. 2001. 203:3–6.
Article8. Campbell DE, Kemp AS. Cyclosporine restores cytokine imbalance in childhood atopic dermatitis. J Allergy Clin Immunol. 1997. 99:857–859.
Article9. Harper JI, Ahmed I, Barclay G, Lacour M, Hoeger P, Cork MJ, et al. Cyclosporin for severe childhood atopic dermatitis: short course versus continuous therapy. Br J Dermatol. 2000. 142:52–58.
Article10. Park C, Kang YS, Lee CH. Immunologic changes after treatment with cyclosporine A in atopic dermatitis. Korean J Dermatol. 1993. 31:210–216.11. Lee SW, Lee YS, Lee SC. The effect of cyclosporin in the treatment of childhood atopic dermatitis. Korean J Dermatol. 2000. 38:466–471.12. Lee JH, Kim KH, Park KC, Chung JH, Suh DH. The efficacy of cyclosporine in patients with severe atopic dermatitis. Ann Dermatol. 2001. 13:12–15.
Article13. Garcia-Bustinduy M, Escoda M, Guimera FJ, Saez M, Dorta S, Fagundo E, et al. Safety of long-term treatment with cyclosporin A in resistant chronic plaque psoriasis: a retrospective case series. J Eur Acad Dermatol Venereol. 2004. 18:169–172.
Article14. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980. 92:44–47.15. Schmid-Grendelmeier P, Simon D, Simon HU, Akdis CA, Wuthrich B. Epidemiology, clinical features, and immunology of the "intrinsic" (non-IgE-mediated) type of atopic dermatitis (constitutional dermatitis). Allergy. 2001. 56:841–849.
Article16. Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology. 1997. 195:10–19.
Article17. Oranje AP, Glazenburg EJ, Wolkerstorfer A, de Waard-van der Spek FB. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007. 157:645–648.
Article18. Lee JS, Yun SJ, Lee JB, Kim SJ, Won YH, Lee SC. The efficacy of cyclosporin in patients with atopic dermatitis. Korean J Dermatol. 2008. 46:224–230.19. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003. 289:2560–2572.
Article20. Leung DYM, Eichenfield LF, Boguniewicz M. Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Atopic dermatitis. Fitzpatrick's dermatology in general medicine. 2008. 7th ed. New York: McGraw-Hill;146–158.21. Bunikowski R, Staab D, Kussebi F, Brautigam M, Weidinger G, Renz H, et al. Low-dose cyclosporin A microemulsion in children with severe atopic dermatitis: clinical and immunological effects. Pediatr Allergy Immunol. 2001. 12:216–223.
Article22. Lee SS, Tan AW, Giam YC. Cyclosporin in the treatment of severe atopic dermatitis: a retrospective study. Ann Acad Med Singapore. 2004. 33:311–313.23. Granlund H, Erkko P, Remitz A, Langeland T, Helsing P, Nuutinen M, et al. Comparison of cyclosporin and UVAB phototherapy for intermittent one-year treatment of atopic dermatitis. Acta Derm Venereol. 2001. 81:22–27.
Article24. Okudaira H, Sakurai Y, Terada K, Terada E, Ogita T, Miyamoto T. Cyclosporin A-induced suppression of ongoing IgE antibody formation in the mouse. Int Arch Allergy Appl Immunol. 1986. 79:164–168.
Article25. Eynott PR, Salmon M, Huang TJ, Oates T, Nicklin PL, Chung KF. Effects of cyclosporin A and a rapamycin derivative (SAR943) on chronic allergic inflammation in sensitized rats. Immunology. 2003. 109:461–467.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Systemic Treatment of Cyclosporin in Severe Atopic Dermatitis
- Vitiligo Lesions Improved after Oral Cyclosporine in a Patient with Vitiligo and Atopic Dermatitis
- A Case of Atopic Dermatitis with Psoriasis
- A Retrospective Analysis of Cyclosporine Dose Regimens and Contributing Factors for Relapse in Atopic Dermatitis
- Therapeutic Efficacy of Mycophenolate Mofetil in Atopic Dermatitis