Ann Dermatol.
2011 Feb;23(1):39-43. 10.5021/ad.2011.23.1.39.
Seroepidemiologic Survey of Varicella-Zoster Virus in Korean Adults Using Glycoprotein Enzyme Immuno Assay and Fluorescent Antibody to Membrane Antigen Test
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Yeungnam University, Daegu, Korea. hspark@med.yu.ac.kr
- 2Department of Dermatology, College of Medicine, Yeungnam University, Daegu, Korea.
- KMID: 2171950
- DOI: http://doi.org/10.5021/ad.2011.23.1.39
Abstract
- BACKGROUND
Herpes zoster (HZ) occurs mainly in the elderly and Korea is rapidly becoming an aging society. Therefore, it is important to know the immune status against varicella-zoster virus (VZV) in Korean adults to prevent the disease.
OBJECTIVE
The aim of this study was to survey the immune status of Korean adults over 40 years of age against VZV.
METHODS
Antibody titer was measured using a VaccZyme(TM) VZV glycoprotein enzyme immunoassay (gpEIA) (Binding Site, UK). Fluorescent antibody to membrane antigen (FAMA) test was performed to measure the seropositive rate.
RESULTS
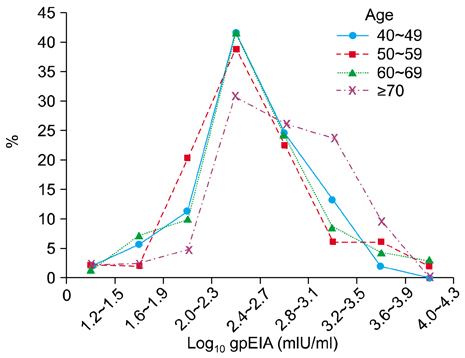
HZ incidence in the 214 adults enrolled in this study was 10.3%. The gpEIA geometric mean titer (GMT) was 490 mIU/ml and 90.2% of the subjects had a protective level of gpEIA antibody titer against varicella. The average gpEIA GMT of adults who previously had HZ was 1,122 mIU/ml, which was higher than the average gpEIA GMT of 457 mIU/ml in adults who had not had HZ. The FAMA positive rate was 98.6%.
CONCLUSION
Most (90.2%) Korean adults > or =40-years-of-age have a protective level of gpEIA antibody against varicella and 98.6% were FAMA seropositive. The GMT of gpEIA antibody was significantly increased with age, and was higher in adults with a history of HZ.
MeSH Terms
Figure
Cited by 1 articles
-
Evaluation of a Commercial Glycoprotein Enzyme-Linked Immunosorbent Assay for Measuring Vaccine Immunity to Varicella
Yun Hwa Kim, Ji Young Hwang, Hye Min Shim, Eunsil Lee, Songyong Park, Hosun Park
Yonsei Med J. 2014;55(2):459-466. doi: 10.3349/ymj.2014.55.2.459.
Reference
-
1. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med. 1965. 58:9–20.
Article2. Arvin AM. Cell-mediated immunity to varicella-zoster virus. J Infect Dis. 1992. 166:Suppl 1. S35–S41.
Article3. Kim HJ, Sung HC, Kim DW, Lee WJ, Lee SJ, Na GY. Clinical features of herpes zoster according to immune state. Korean J Dermatol. 2006. 44:149–156.4. Kim CG, Na CH, Choi KC, Shin BS. A comparative study of the clinical findings associated with herpes zoster and according to age. Korean J Dermatol. 2009. 47:1338–1344.5. Harpaz R, Ortega-Sanchez IR, Seward JF. Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008. 57:1–30.6. Williams V, Gershon A, Brunell PA. Serologic response to varicella-zoster membrane antigens measured by direct immunofluorescence. J Infect Dis. 1974. 130:669–672.
Article7. de Ory F, Echevarría JM, Kafatos G, Anastassopoulou C, Andrews N, Backhouse J, et al. European seroepidemiology network 2: Standardisation of assays for seroepidemiology of varicella zoster virus. J Clin Virol. 2006. 36:111–118.
Article8. Provost PJ, Krah DL, Kuter BJ, Morton DH, Schofield TL, Wasmuth EH, et al. Antibody assays suitable for assessing immune responses to live varicella vaccine. Vaccine. 1991. 9:111–116.
Article9. Krah DL, Cho I, Schofield T, Ellis RW. Comparison of gpELISA and neutralizing antibody responses to Oka/Merck live varicella vaccine (Varivax) in children and adults. Vaccine. 1997. 15:61–64.
Article10. Shin HS, Oh HS, Kim SM, Kim NJ, Choi HJ, Oh MD, et al. Prevalence of measles, rubella and varicella-zoster antibodies in hospital personnel. Korean J Infect Dis. 1997. 29:29–32.11. Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995. 155:1605–1609.
Article12. Schmader K, George LK, Burchett BM, Pieper CF, Hamilton JD. Racial differences in the occurrence of herpes zoster. J Infect Dis. 1995. 171:701–704.
Article13. Helgason S, Sigurdsson JA, Gudmundsson S. The clinical course of herpes zoster: a prospective study in primary care. Eur J Gen Pract. 1996. 2:12–16.
Article14. Ragozzino MW, Melton LJ 3rd, Kurland LT, Chu CP, Perry HO. Population-based study of herpes zoster and its sequelae. Medicine (Baltimore). 1982. 61:310–316.
Article15. Yawn BP, Saddier P, Wollan PC, St Sauver JL, Kurland MJ, Sy LS. A population-based study of the incidence and complication rates of herpes zoster before zoster vaccine introduction. Mayo Clin Proc. 2007. 82:1341–1349.
Article16. Sadaoka K, Okamoto S, Gomi Y, Tanimoto T, Ishikawa T, Yoshikawa T, et al. Measurement of varicella-zoster virus (VZV)-specific cell-mediated immunity: comparison between VZV skin test and interferon-gamma enzyme-linked immunospot assay. J Infect Dis. 2008. 198:1327–1333.
Article17. Weinberg A, Zhang JH, Oxman MN, Johnson GR, Hayward AR, Caulfield MJ, et al. Varicella-zoster virus-specific immune responses to herpes zoster in elderly participants in a trial of a clinically effective zoster vaccine. J Infect Dis. 2009. 200:1068–1077.
Article18. Levin MJ, Oxman MN, Zhang JH, Johnson GR, Stanley H, Hayward AR, et al. Varicella-zoster virus-specific immune responses in elderly recipients of a herpes zoster vaccine. J Infect Dis. 2008. 197:825–835.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation Methods for the Immunogenicity of Varicella and Zoster Vaccines
- Humoral and Cell Mediated Immune Response After Immunization with Varicella Vaccine (Oka/LG)
- Safety and Immunogenicity of Live Attenuated Varicella Virus Vaccine(MAV/06 Strain)
- The usefulness of skin test in evaluation of immunity to varicella
- Prevalence of Measles, Rubella and Varicella-Zoster Antibodies in Hospital Personnel