Ewha Med J.
2016 Apr;39(2):37-44. 10.12771/emj.2016.39.2.37.
Diagnosis for Imported Cases of Emerging and Reemerging Infectious Diseases in Korea
- Affiliations
-
- 1Department of Laboratory Medicine, Ewha Womans University School of Medicine, Seoul, Korea. miae@ewha.ac.kr
- KMID: 2171363
- DOI: http://doi.org/10.12771/emj.2016.39.2.37
Abstract
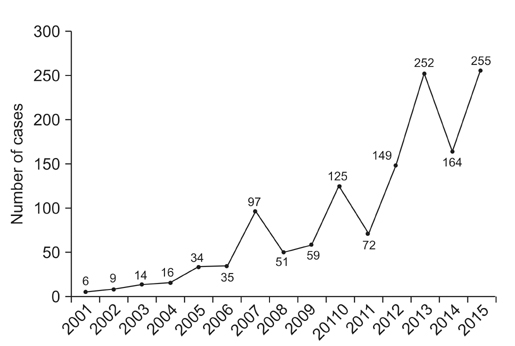
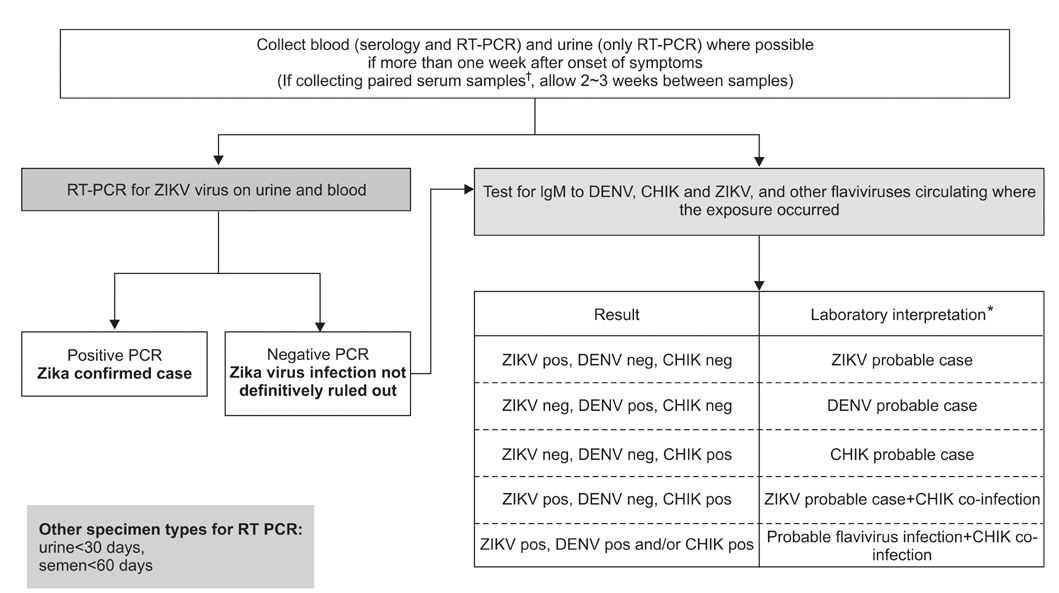
- Despite recent advances in the development of diagnostics, therapeutics, and vaccines, the ease of international travel and increasing global interdependence have brought about particular challenges for the control of infectious diseases, highlighting concerns for the worldwide spread of emerging and reemerging infectious diseases. Korea is also facing public health challenges for controlling imported cases of infectious diseases; dengue virus, which is the most commonly reported case of imported infectious diseases; the largest outbreak of Middle East respiratory syndrome coronavirus infections outside the Arabian Peninsula in 2015; and the Zika virus infection, which was declared by the WHO as a "Public Health Emergency of International Concern." Although national and global partnerships are critical to controlling imported infectious disease threats, the role of local hospitals, public health sectors, and laboratory capacity remains the cornerstone for initial disease recognition and response. The current status of laboratory diagnosis for imported infectious diseases is reviewed.
Keyword
MeSH Terms
Figure
Reference
-
1. Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, et al. Global trends in emerging infectious diseases. Nature. 2008; 451:990–993.2. Khabbaz R, Bell BP, Schuchat A, Ostroff SM, Moseley R, Levitt A, et al. Emerging and reemerging infectious disease treats. In : Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 8th ed. Philadelphia: Churchill Livingstone;2015. p. 158–177.3. World Health Organization. Severe acute respiratory syndrome (SARS) multi-country outbreak: update 6 [Internet]. Geneva: World Health Organization;2003. cited 2016 Apr 10. Available from: http://www.who.int/csr/don/2003_03_21/en/index.html.4. Centers for Disease Control and Prevention. The 2009 H1N1 pandemic: summary highlights, April 2009-April 2010 [Internet]. Atlanta, GA: Centers for Disease Control and Prevention;2010. cited 2016 Apr 10. Available from: http://www.cdc.gov/h1n1flu/cdcresponse.htm.5. WHO Ebola Response Team. Ebola virus disease in West Africa: the first 9 months of the epidemic and forward projections. N Engl J Med. 2014; 371:1481–1495.6. World Health Organization. WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations [Internet]. Geneva: World Health Organization;2016. cited 2016 Feb 1. Available from: http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/.7. World Health Organization. International Health Regulations (2005) [Internet]. 2nd ed. Geneva: World Health Organization;2008. cited 2016 Apr 10. Available from: http://apps.who.int/iris/bitstream/10665/43883/1/9789241580410_eng.pdf.8. Korea Centers for Disease Control and Prevention. Infectious diseases surveillance yearbook. Cheongju, KR: Korea Centers for Disease Control and Prevention;2014.9. Korea Centers for Disease Control and Prevention. Infectious Disease Statistics System [Internet]. Cheongju: Korea Centers for Disease Control and Prevention;2016. cited 2016 Mar 16. Available from: http://is.cdc.go.kr/dstat/index.jsp.10. Yu XJ, Liang MF, Zhang SY, Liu Y, Li JD, Sun YL, et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N Engl J Med. 2011; 364:1523–1532.11. Kim KH, Yi J, Kim G, Choi SJ, Jun KI, Kim NH, et al. Severe fever with thrombocytopenia syndrome, South Korea, 2012. Emerg Infect Dis. 2013; 19:1892–1894.12. Thomas SJ, Endy TP, Rothman AL, Barrett AD. Flaviviruses. In : Bennett JE, Dolin R, Blaser MJ, editors. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia: Churchill Livingstone;2015. p. 1881–1903.13. Guzman MG, Harris E. Dengue. Lancet. 2015; 385:453–465.14. World Health Organization. Global strategy for dengue prevention and control. Geneva: World Health Organization;2012.15. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013; 496:504–507.16. Deen JL, Harris E, Wills B, Balmaseda A, Hammond SN, Rocha C, et al. The WHO dengue classification and case definitions: time for a reassessment. Lancet. 2006; 368:170–173.17. World Health Organization. Dengue hemorrhagic fever: diagnosis, treatment, prevention, and control. 2nd ed. Geneva: World Health Organization;1997.18. World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control [Internet]. Geneva: World Health Organization;2009. cited 2016 Mar 16. Available from: http://www.who.int/tdr/publications/documents/dengue-diagnosis.pdf.19. Santiago GA, Vergne E, Quiles Y, Cosme J, Vazquez J, Medina JF, et al. Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl Trop Dis. 2013; 7:e2311.20. Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, et al. Comparison of capture immunoglobulin M (IgM) and IgG enzyme-linked immunosorbent assay (ELISA) and nonstructural protein NS1 serotype-specific IgG ELISA for differentiation of primary and secondary dengue virus infections. Clin Diagn Lab Immunol. 2003; 10:622–630.21. Falconar AK, de Plata E, Romero-Vivas CM. Altered enzymelinked immunosorbent assay immunoglobulin M (IgM)/IgG optical density ratios can correctly classify all primary or secondary dengue virus infections 1 day after the onset of symptoms, when all of the viruses can be isolated. Clin Vaccine Immunol. 2006; 13:1044–1051.22. Bhat VG, Chavan P, Ojha S, Nair PK. Challenges in the laboratory diagnosis and management of dengue infections. Open Microbiol J. 2015; 9:33–37.23. Huang CH, Kuo LL, Yang KD, Lin PS, Lu PL, Lin CC, et al. Laboratory diagnostics of dengue fever: an emphasis on the role of commercial dengue virus nonstructural protein 1 antigen rapid test. J Microbiol Immunol Infect. 2013; 46:358–365.24. Huhtamo E, Hasu E, Uzcategui NY, Erra E, Nikkari S, Kantele A, et al. Early diagnosis of dengue in travelers: comparison of a novel real-time RT-PCR, NS1 antigen detection and serology. J Clin Virol. 2010; 47:49–53.25. Jeong YE, Lee EJ. Current status of dengue diagnostic laboratory in the Republic of Korea. Public Health Wkly Rep. 2013; 6:761–765.26. Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012; 367:1814–1820.27. Rha B, Rudd J, Feikin D, Watson J, Curns AT, Swerdlow DL, et al. Update on the epidemiology of Middle East respiratory syndrome coronavirus (MERS-CoV) infection, and guidance for the public, clinicians, and public health authorities - January 2015. MMWR Morb Mortal Wkly Rep. 2015; 64:61–62.28. Ki M. 2015 MERS outbreak in Korea: hospital-to-hospital transmission. Epidemiol Health. 2015; 37:e2015033.29. Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, et al. Evidence for camel-to-human transmission of MERS coronavirus. N Engl J Med. 2014; 370:2499–2505.30. World Health Organization. Laboratory testing for Middle East respiratory syndrome coronavirus (MERS-CoV): interim guidance [Internet]. Geneva: World Health Organization;2015. cited 2016 Apr 10. Available from: http://www.who.int/csr/disease/coronavirus_infections/mers-laboratory-testing/en/.31. Corman VM, Muller MA, Costabel U, Timm J, Binger T, Meyer B, et al. Assays for laboratory confirmation of novel human coronavirus (hCoV-EMC) infections. Euro Surveill. 2012; 17:20334.32. Corman VM, Eckerle I, Bleicker T, Zaki A, Landt O, Eschbach-Bludau M, et al. Detection of a novel human coronavirus by real-time reverse-transcription polymerase chain reaction. Euro Surveill. 2012; 17:20285.33. Lee MK, Kim S, Kim MN, Kweon OJ, Lim YK, Ki CS, et al. Survey of clinical laboratory practices for 2015 middle east respiratory syndrome coronavirus outbreak in the republic of Korea. Ann Lab Med. 2016; 36:154–161.34. Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015; 110:569–572.35. World Health Organization. Zika virus disease: interim case definition [Internet]. Geneva: World Health Organization;2015. cited 2016 Feb 16. Available from: http://www.who.int/csr/disease/zika/case-definition/en/.36. Petersen E, Wilson ME, Touch S, McCloskey B, Mwaba P, Bates M, et al. Rapid spread of Zika virus in the americas - implications for public health preparedness for mass gatherings at the 2016 Brazil olympic games. Int J Infect Dis. 2016; 44:11–15.37. Foy BD, Kobylinski KC, Chilson Foy JL, Blitvich BJ, Travassos da Rosa A, Haddow AD, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis. 2011; 17:880–882.38. Musso D, Roche C, Robin E, Nhan T, Teissier A, Cao-Lormeau VM. Potential sexual transmission of Zika virus. Emerg Infect Dis. 2015; 21:359–361.39. Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, et al. Zika virus: high infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016; 16:405.40. Musso D, Nhan T, Robin E, Roche C, Bierlaire D, Zisou K, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014; 19:20761.41. Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill. 2014; 19:20751.42. Oliveira Melo AS, Malinger G, Ximenes R, Szejnfeld PO, Alves Sampaio S, Bispo de Filippis AM. Zika virus intrauterine infection causes fetal brain abnormality and microcephaly: tip of the iceberg. Ultrasound Obstet Gynecol. 2016; 47:6–7.43. Centers for Disease Control and Prevention. Zika virus: diagnostic testing [Internet]. Atlanta, GA: Centers for Disease Control and Prevention;2016. cited 2016 Mar 22. Available from: http://www.cdc.gov/zika/hc-providers/diagnostic.html.44. World Health Organization. Laboratory testing for Zika virus infection: interim guidance [Internet]. Geneva: World Health Organization;2016. cited 2016 Mar 23. Available from: http://www.who.int/csr/resources/publications/zika/laboratory-testing/en/.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- International Cooperation in the Control and Prevention of Emerging and Reemerging Infectious Diseases
- Reemerging Old Infectious Diseases: Diagnosis of Measles, Mumps, Rubella, and Pertussis
- The Role of Epidemiology against Emerging and Reemerging Diseases
- Surveillance System for Communicable Disease in Korea
- Emerging and Reemerging Infections in Children