Infect Chemother.
2016 Mar;48(1):31-35. 10.3947/ic.2016.48.1.31.
Fecal Transplantation using a Nasoenteric Tube during an Initial Episode of Severe Clostridium difficile Infection
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea. seran@yuhs.ac
- 2AIDS Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Laboratory Medicine, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2170514
- DOI: http://doi.org/10.3947/ic.2016.48.1.31
Abstract
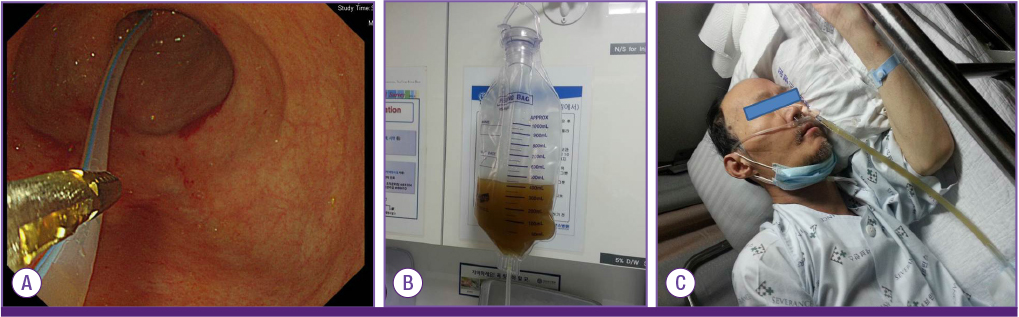
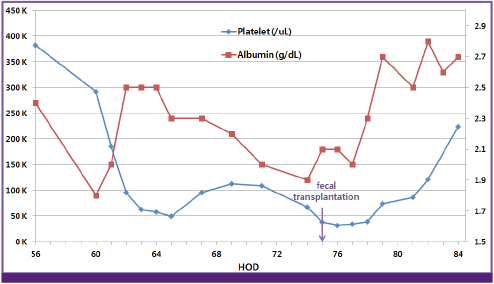
- The incidence of Clostridium difficile infection is increasing worldwide, and its severity and resulting mortality are also on the rise. Metronidazole and oral vancomycin remain the treatments of choice, but there are concerns about treatment failure and the appearance of resistant strains. Furthermore, antibiotic therapy results in recurrence rates of at least 20%. Fecal transplantation may be a feasible treatment option for recurrent C. difficile infection; moreover, it may be an early treatment option for severe C. difficile infection. We report a case of severe C. difficile infection treated with fecal transplantation using a nasoenteric tube during an initial episode. This is the first reported case of fecal transplantation using a nasoenteric tube during an initial episode of C. difficile infection in Korea.
MeSH Terms
Figure
Cited by 1 articles
-
Fecal Microbiota Transplantation for Refractory and Recurrent Clostridium difficile Infection: A Case Series of Nine Patients
Byoung Wook Bang, Jin-Seok Park, Hyung Kil Kim, Yong Woon Shin, Kye Sook Kwon, Hea Yoon Kwon, Ji Hyeon Baek, Jin-Soo Lee
Korean J Gastroenterol. 2017;69(4):226-231. doi: 10.4166/kjg.2017.69.4.226.
Reference
-
1. Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, Pépin K, Chouinard D. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004; 171:466–472.2. Loo VG, Poirier L, Miller MA, Oughton M, Libman MD, Michaud S, Bourgault AM, Nguyen T, Frenette C, Kelly M, Vibien A, Brassard P, Fenn S, Dewar K, Hudson TJ, Horn R, René P, Monczak Y, Dascal A. A predominantly clonal multi-institutional outbreak of Clostridium difficile-associated diarrhea with high morbidity and mortality. N Engl J Med. 2005; 353:2442–2449.
Article3. Muto CA, Pokrywka M, Shutt K, Mendelsohn AB, Nouri K, Posey K, Roberts T, Croyle K, Krystofiak S, Patel-Brown S, Pasculle AW, Paterson DL, Saul M, Harrison LH. A large outbreak of Clostridium difficile-associated disease with an unexpected proportion of deaths and colectomies at a teaching hospital following increased fluoroquinolone use. Infect Control Hosp Epidemiol. 2005; 26:273–280.
Article4. Kelly CP, LaMont JT. Clostridium difficile--more difficult than ever. N Engl J Med. 2008; 359:1932–1940.5. Rineh A, Kelso MJ, Vatansever F, Tegos GP, Hamblin MR. Clostridium difficile infection: molecular pathogenesis and novel therapeutics. Expert Rev Anti Infect Ther. 2014; 12:131–150.
Article6. Borody TJ, Paramsothy S, Agrawal G. Fecal microbiota transplantation: indications, methods, evidence, and future directions. Curr Gastroenterol Rep. 2013; 15:337.
Article7. van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013; 368:407–415.
Article8. Borody TJ, Peattie D, Kapur A. Could fecal microbiota transplantation cure all Clostridium difficile infections? Future Microbiol. 2014; 9:1–3.
Article9. Gweon TG, Choi MG, Lee SK, Ha JH, Kim EY, Go BS, Kim SW. Two cases of refractory Pseudomembranous colitis that healed following fecal microbiota transplantation. Korean J Med. 2013; 84:395–399.
Article10. Moon KR, Sohn KM, Park BM, Kim YS, Chun S, Jung H, Song CH. Successful fecal transplantation by enema for recurrent and refractory Clostridium difficile infection. J Korean Geriatr Soc. 2013; 17:152–156.
Article11. McCune VL, Struthers JK, Hawkey PM. Faecal transplantation for the treatment of Clostridium difficile infection: a review. Int J Antimicrob Agents. 2014; 43:201–206.
Article12. Brandt LJ, Aroniadis OC. An overview of fecal microbiota transplantation: techniques, indications, and outcomes. Gastrointest Endosc. 2013; 78:240–249.
Article13. Zainah H, Hassan M, Shiekh-Sroujieh L, Hassan S, Alangaden G, Ramesh M. Intestinal microbiota transplantation, a simple and effective treatment for severe and refractory Clostridium difficile infection. Dig Dis Sci. 2015; 60:181–185.
Article14. Brandt LJ, Borody TJ, Campbell J. Endoscopic fecal microbiota transplantation: "first-line" treatment for severe Clostridium difficile infection? J Clin Gastroenterol. 2011; 45:655–657.15. Gough E, Shaikh H, Manges AR. Systematic review of intestinal microbiota transplantation (fecal bacteriotherapy) for recurrent Clostridium difficile infection. Clin Infect Dis. 2011; 53:994–1002.
Article16. Postigo R, Kim JH. Colonoscopic versus nasogastric fecal transplantation for the treatment of Clostridium difficile infection: a review and pooled analysis. Infection. 2012; 40:643–648.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Toxic Megacolon Caused by Clostridium difficile Infection and Treated with Fecal Microbiota Transplantation
- Refractory Clostridium difficile Infection Cured With Fecal Microbiota Transplantation in Vancomycin-Resistant Enterococcus Colonized Patient
- Fecal Microbiota Transplantation as a Treatment of Recurrent Clostridium difficile Infection: Where Are We Now and Where Are We Heading?
- Fecal Bacteriotherapy for Recurrent Clostridium difficile Infection: A Systematic Literature Review
- Successful Fecal Transplantation by Enema for Recurrent and Refractory Clostridium difficile Infection