Endocrinol Metab.
2013 Dec;28(4):262-274. 10.3803/EnM.2013.28.4.262.
Clinical Application of Glucagon-Like Peptide 1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus
- Affiliations
-
- 1Division of Endocrinology and Metabolism, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 2Laboratory of Molecular and Cellular Medicine, Departments of Cellular and Physiological Sciences and Surgery, Life Sciences Institute, University of British Columbia, Vancouver, BC, Canada. tim.kieffer@ubc.ca
- KMID: 2169303
- DOI: http://doi.org/10.3803/EnM.2013.28.4.262
Abstract
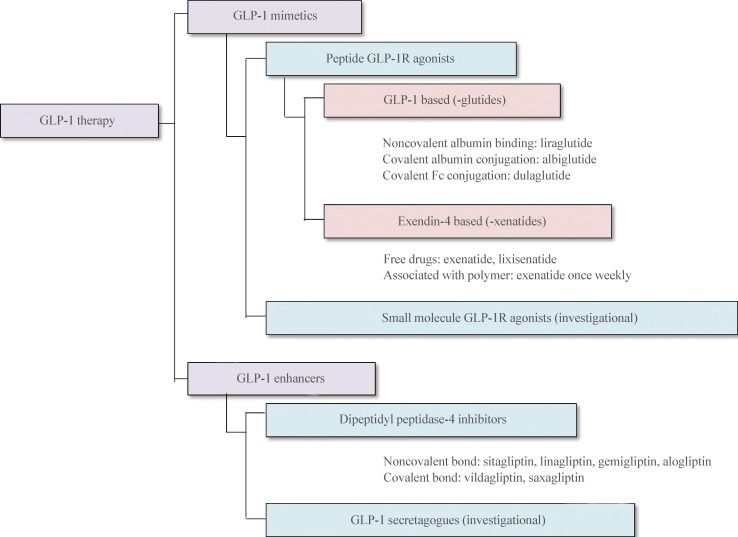
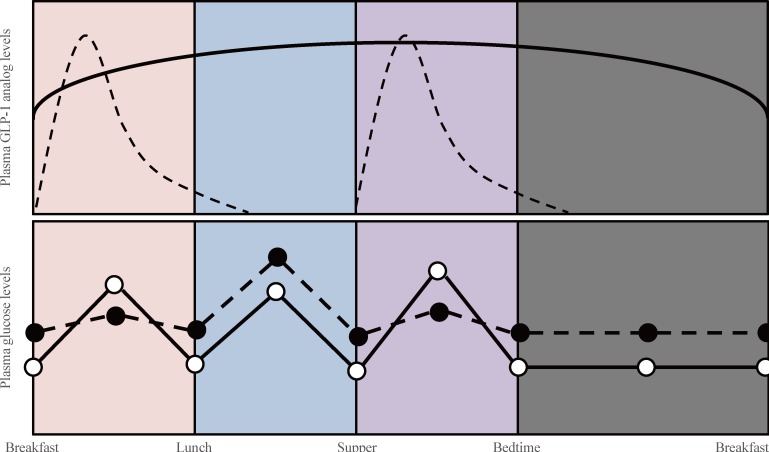
- Glucagon-like peptide 1 (GLP-1) is secreted from enteroendocrine L-cells in response to oral nutrient intake and elicits glucose-stimulated insulin secretion while suppressing glucagon secretion. It also slows gastric emptying, which contributes to decreased postprandial glycemic excursions. In the 1990s, chronic subcutaneous infusion of GLP-1 was found to lower blood glucose levels in patients with type 2 diabetes. However, GLP-1's very short half-life, arising from cleavage by the enzyme dipeptidyl peptidase 4 (DPP-4) and glomerular filtration by the kidneys, presented challenges for clinical use. Hence, DPP-4 inhibitors were developed, as well as several GLP-1 analogs engineered to circumvent DPP-4-mediated breakdown and/or rapid renal elimination. Three categories of GLP-1 analogs, are being developed and/or are in clinical use: short-acting, long-acting, and prolonged-acting GLP-1 analogs. Each class has different plasma half-lives, molecular size, and homology to native GLP-1, and consequently different characteristic effects on glucose metabolism. In this article, we review current clinical data derived from each class of GLP-1 analogs, and consider the clinical effects reported for each category in recent head to head comparison studies. Given the relatively brief clinical history of these compounds, we also highlight several important efficacy and safety issues which will require further investigation.
Keyword
MeSH Terms
-
Blood Glucose
Diabetes Mellitus, Type 2*
Dipeptidyl Peptidase 4
Filtration
Gastric Emptying
Glucagon
Glucagon-Like Peptide 1*
Glucose
Half-Life
Head
Humans
Infusions, Subcutaneous
Insulin
Kidney
Metabolism
Peptides
Plasma
Venoms
Liraglutide
Blood Glucose
Dipeptidyl Peptidase 4
Glucagon
Glucagon-Like Peptide 1
Glucose
Insulin
Peptides
Venoms
Figure
Cited by 3 articles
-
Brief Review of Articles in '
Endocrinology and Metabolism ' in 2013
Won-Young Lee
Endocrinol Metab. 2014;29(3):251-256. doi: 10.3803/EnM.2014.29.3.251.Clinical Application of Glucagon-Like Peptide-1 Receptor Agonists
Se Hee Min, Young Min Cho
J Korean Diabetes. 2015;16(4):252-259. doi: 10.4093/jkd.2015.16.4.252.Peptidyl and Non-Peptidyl Oral Glucagon-Like Peptide-1 Receptor Agonists
Hun Jee Choe, Young Min Cho
Endocrinol Metab. 2021;36(1):22-29. doi: 10.3803/EnM.2021.102.
Reference
-
1. Gutniak M, Orskov C, Holst JJ, Ahren B, Efendic S. Antidiabetogenic effect of glucagon-like peptide-1 (7-36)amide in normal subjects and patients with diabetes mellitus. N Engl J Med. 1992; 326:1316–1322. PMID: 1348845.
Article2. Nathan DM, Schreiber E, Fogel H, Mojsov S, Habener JF. Insulinotropic action of glucagonlike peptide-I-(7-37) in diabetic and nondiabetic subjects. Diabetes Care. 1992; 15:270–276. PMID: 1547685.
Article3. Nauck MA, Kleine N, Orskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7-36 amide) in type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993; 36:741–744. PMID: 8405741.
Article4. Zander M, Madsbad S, Madsen JL, Holst JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet. 2002; 359:824–830. PMID: 11897280.5. Deacon CF, Johnsen AH, Holst JJ. Degradation of glucagon-like peptide-1 by human plasma in vitro yields an N-terminally truncated peptide that is a major endogenous metabolite in vivo. J Clin Endocrinol Metab. 1995; 80:952–957. PMID: 7883856.
Article6. Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. 1995; 44:1126–1131. PMID: 7657039.
Article7. Kieffer TJ, McIntosh CH, Pederson RA. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology. 1995; 136:3585–3596. PMID: 7628397.
Article8. Vilsboll T, Agerso H, Krarup T, Holst JJ. Similar elimination rates of glucagon-like peptide-1 in obese type 2 diabetic patients and healthy subjects. J Clin Endocrinol Metab. 2003; 88:220–224. PMID: 12519856.9. Bush MA, Matthews JE, De Boever EH, Dobbins RL, Hodge RJ, Walker SE, Holland MC, Gutierrez M, Stewart MW. Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes Obes Metab. 2009; 11:498–505. PMID: 19187286.
Article10. Nauck MA, Ratner RE, Kapitza C, Berria R, Boldrin M, Balena R. Treatment with the human once-weekly glucagon-like peptide-1 analog taspoglutide in combination with metformin improves glycemic control and lowers body weight in patients with type 2 diabetes inadequately controlled with metformin alone: a double-blind placebo-controlled study. Diabetes Care. 2009; 32:1237–1243. PMID: 19366970.11. Leger R, Thibaudeau K, Robitaille M, Quraishi O, van Wyk P, Bousquet-Gagnon N, Carette J, Castaigne JP, Bridon DP. Identification of CJC-1131-albumin bioconjugate as a stable and bioactive GLP-1(7-36) analog. Bioorg Med Chem Lett. 2004; 14:4395–4398. PMID: 15357960.12. Knudsen LB, Nielsen PF, Huusfeldt PO, Johansen NL, Madsen K, Pedersen FZ, Thogersen H, Wilken M, Agerso H. Potent derivatives of glucagon-like peptide-1 with pharmacokinetic properties suitable for once daily administration. J Med Chem. 2000; 43:1664–1669. PMID: 10794683.
Article13. Meier JJ, Nauck MA, Kranz D, Holst JJ, Deacon CF, Gaeckler D, Schmidt WE, Gallwitz B. Secretion, degradation, and elimination of glucagon-like peptide 1 and gastric inhibitory polypeptide in patients with chronic renal insufficiency and healthy control subjects. Diabetes. 2004; 53:654–662. PMID: 14988249.
Article14. Werner U, Haschke G, Herling AW, Kramer W. Pharmacological profile of lixisenatide: a new GLP-1 receptor agonist for the treatment of type 2 diabetes. Regul Pept. 2010; 164:58–64. PMID: 20570597.
Article15. Barrington P, Chien JY, Showalter HD, Schneck K, Cui S, Tibaldi F, Ellis B, Hardy TA. A 5-week study of the pharmacokinetics and pharmacodynamics of LY2189265, a novel, long-acting glucagon-like peptide-1 analogue, in patients with type 2 diabetes. Diabetes Obes Metab. 2011; 13:426–433. PMID: 21251178.
Article16. Glaesner W, Vick AM, Millican R, Ellis B, Tschang SH, Tian Y, Bokvist K, Brenner M, Koester A, Porksen N, Etgen G, Bumol T. Engineering and characterization of the long-acting glucagon-like peptide-1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010; 26:287–296. PMID: 20503261.
Article17. Drucker DJ, Buse JB, Taylor K, Kendall DM, Trautmann M, Zhuang D, Porter L. DURATION-1 Study Group. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008; 372:1240–1250. PMID: 18782641.
Article18. Henry RR, Rosenstock J, Logan DK, Alessi TR, Luskey K, Baron MA. Randomized trial of continuous subcutaneous delivery of exenatide by ITCA 650 versus twice-daily exenatide injections in metformin-treated type 2 diabetes. Diabetes Care. 2013; 36(9):2559–2565. PMID: 23645886.
Article19. Buse JB, Nauck M, Forst T, Sheu WH, Shenouda SK, Heilmann CR, Hoogwerf BJ, Gao A, Boardman MK, Fineman M, Porter L, Schernthaner G. Exenatide once weekly versus liraglutide once daily in patients with type 2 diabetes (DURATION-6): a randomised, open-label study. Lancet. 2013; 381:117–124. PMID: 23141817.
Article20. Cho YM, Merchant CE, Kieffer TJ. Targeting the glucagon receptor family for diabetes and obesity therapy. Pharmacol Ther. 2012; 135:247–278. PMID: 22659620.
Article21. Chen D, Liao J, Li N, Zhou C, Liu Q, Wang G, Zhang R, Zhang S, Lin L, Chen K, Xie X, Nan F, Young AA, Wang MW. A nonpeptidic agonist of glucagon-like peptide 1 receptors with efficacy in diabetic db/db mice. Proc Natl Acad Sci U S A. 2007; 104:943–948. PMID: 17213311.22. Su H, He M, Li H, Liu Q, Wang J, Wang Y, Gao W, Zhou L, Liao J, Young AA, Wang MW. Boc5, a non-peptidic glucagon-like Peptide-1 receptor agonist, invokes sustained glycemic control and weight loss in diabetic mice. PLoS One. 2008; 3:e2892. PMID: 18682834.
Article23. He M, Guan N, Gao WW, Liu Q, Wu XY, Ma DW, Zhong DF, Ge GB, Li C, Chen XY, Yang L, Liao JY, Wang MW. A continued saga of Boc5, the first non-peptidic glucagon-like peptide-1 receptor agonist with in vivo activities. Acta Pharmacol Sin. 2012; 33:148–154. PMID: 22301855.
Article24. Deacon CF. Dipeptidyl peptidase-4 inhibitors in the treatment of type 2 diabetes: a comparative review. Diabetes Obes Metab. 2011; 13:7–18. PMID: 21114598.
Article25. Drucker DJ. Dipeptidyl peptidase-4 inhibition and the treatment of type 2 diabetes: preclinical biology and mechanisms of action. Diabetes Care. 2007; 30:1335–1343. PMID: 17337495.26. Reimann F, Habib AM, Tolhurst G, Parker HE, Rogers GJ, Gribble FM. Glucose sensing in L cells: a primary cell study. Cell Metab. 2008; 8:532–539. PMID: 19041768.
Article27. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, Macchiarulo A, Yamamoto H, Mataki C, Pruzanski M, Pellicciari R, Auwerx J, Schoonjans K. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009; 10:167–177. PMID: 19723493.
Article28. Lan H, Vassileva G, Corona A, Liu L, Baker H, Golovko A, Abbondanzo SJ, Hu W, Yang S, Ning Y, Del Vecchio RA, Poulet F, Laverty M, Gustafson EL, Hedrick JA, Kowalski TJ. GPR119 is required for physiological regulation of glucagon-like peptide-1 secretion but not for metabolic homeostasis. J Endocrinol. 2009; 201:219–230. PMID: 19282326.
Article29. Nauck MA, Meier JJ. Individualised incretin-based treatment for type 2 diabetes. Lancet. 2010; 376:393–394. PMID: 20580425.
Article30. Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012; 8:728–742. PMID: 22945360.
Article31. Esposito K, Mosca C, Brancario C, Chiodini P, Ceriello A, Giugliano D. GLP-1 receptor agonists and HBA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Curr Med Res Opin. 2011; 27:1519–1528. PMID: 21663496.32. Vilsboll T, Christensen M, Junker AE, Knop FK, Gluud LL. Effects of glucagon-like peptide-1 receptor agonists on weight loss: systematic review and meta-analyses of randomised controlled trials. BMJ. 2012; 344:d7771. PMID: 22236411.
Article33. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012; 33:187–215. PMID: 22323472.
Article34. Fineman MS, Bicsak TA, Shen LZ, Taylor K, Gaines E, Varns A, Kim D, Baron AD. Effect on glycemic control of exenatide (synthetic exendin-4) additive to existing metformin and/or sulfonylurea treatment in patients with type 2 diabetes. Diabetes Care. 2003; 26:2370–2377. PMID: 12882864.
Article35. Kolterman OG, Kim DD, Shen L, Ruggles JA, Nielsen LL, Fineman MS, Baron AD. Pharmacokinetics, pharmacodynamics, and safety of exenatide in patients with type 2 diabetes mellitus. Am J Health Syst Pharm. 2005; 62:173–181. PMID: 15700891.
Article36. Simonsen L, Holst JJ, Deacon CF. Exendin-4, but not glucagon-like peptide-1, is cleared exclusively by glomerular filtration in anaesthetised pigs. Diabetologia. 2006; 49:706–712. PMID: 16447056.
Article37. Tourrel C, Bailbe D, Lacorne M, Meile MJ, Kergoat M, Portha B. Persistent improvement of type 2 diabetes in the Goto-Kakizaki rat model by expansion of the beta-cell mass during the prediabetic period with glucagon-like peptide-1 or exendin-4. Diabetes. 2002; 51:1443–1452. PMID: 11978641.38. Tourrel C, Bailbe D, Meile MJ, Kergoat M, Portha B. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes. 2001; 50:1562–1570. PMID: 11423477.39. Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999; 48:2270–2276. PMID: 10580413.
Article40. Fehse F, Trautmann M, Holst JJ, Halseth AE, Nanayakkara N, Nielsen LL, Fineman MS, Kim DD, Nauck MA. Exenatide augments first- and second-phase insulin secretion in response to intravenous glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2005; 90:5991–5997. PMID: 16144950.
Article41. Kolterman OG, Buse JB, Fineman MS, Gaines E, Heintz S, Bicsak TA, Taylor K, Kim D, Aisporna M, Wang Y, Baron AD. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003; 88:3082–3089. PMID: 12843147.
Article42. Linnebjerg H, Park S, Kothare PA, Trautmann ME, Mace K, Fineman M, Wilding I, Nauck M, Horowitz M. Effect of exenatide on gastric emptying and relationship to postprandial glycemia in type 2 diabetes. Regul Pept. 2008; 151:123–129. PMID: 18675854.
Article43. Tahrani AA, Piya MK, Kennedy A, Barnett AH. Glycaemic control in type 2 diabetes: targets and new therapies. Pharmacol Ther. 2010; 125:328–361. PMID: 19931305.
Article44. Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG. GWAA Study Group. Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005; 143:559–569. PMID: 16230722.45. Buse JB, Bergenstal RM, Glass LC, Heilmann CR, Lewis MS, Kwan AY, Hoogwerf BJ, Rosenstock J. Use of twice-daily exenatide in Basal insulin-treated patients with type 2 diabetes: a randomized, controlled trial. Ann Intern Med. 2011; 154:103–112. PMID: 21138825.46. Klonoff DC, Buse JB, Nielsen LL, Guan X, Bowlus CL, Holcombe JH, Wintle ME, Maggs DG. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr Med Res Opin. 2008; 24:275–286. PMID: 18053320.
Article47. Gallwitz B, Guzman J, Dotta F, Guerci B, Simo R, Basson BR, Festa A, Kiljanski J, Sapin H, Trautmann M, Schernthaner G. Exenatide twice daily versus glimepiride for prevention of glycaemic deterioration in patients with type 2 diabetes with metformin failure (EUREXA): an open-label, randomised controlled trial. Lancet. 2012; 379:2270–2278. PMID: 22683137.
Article48. Riddle MC, Henry RR, Poon TH, Zhang B, Mac SM, Holcombe JH, Kim DD, Maggs DG. Exenatide elicits sustained glycaemic control and progressive reduction of body weight in patients with type 2 diabetes inadequately controlled by sulphonylureas with or without metformin. Diabetes Metab Res Rev. 2006; 22:483–491. PMID: 16634116.
Article49. Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Exenatide-113 Clinical Study Group. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004; 27:2628–2635. PMID: 15504997.
Article50. DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005; 28:1092–1100. PMID: 15855572.
Article51. Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005; 28:1083–1091. PMID: 15855571.
Article52. Buse JB, Garber A, Rosenstock J, Schmidt WE, Brett JH, Videbaek N, Holst J, Nauck M. Liraglutide treatment is associated with a low frequency and magnitude of antibody formation with no apparent impact on glycemic response or increased frequency of adverse events: results from the Liraglutide Effect and Action in Diabetes (LEAD) trials. J Clin Endocrinol Metab. 2011; 96:1695–1702. PMID: 21450987.
Article53. Butler PC, Elashoff M, Elashoff R, Gale EA. A critical analysis of the clinical use of incretin-based therapies: are the GLP-1 therapies safe? Diabetes Care. 2013; 36:2118–2125. PMID: 23645885.54. Nauck MA. A critical analysis of the clinical use of incretin-based therapies: the benefits by far outweigh the potential risks. Diabetes Care. 2013; 36:2126–2132. PMID: 23645884.55. Christensen M, Knop FK, Vilsboll T, Holst JJ. Lixisenatide for type 2 diabetes mellitus. Expert Opin Investig Drugs. 2011; 20:549–557.
Article56. Ratner RE, Rosenstock J, Boka G. DRI6012 Study Investigators. Dose-dependent effects of the once-daily GLP-1 receptor agonist lixisenatide in patients with type 2 diabetes inadequately controlled with metformin: a randomized, double-blind, placebo-controlled trial. Diabet Med. 2010; 27:1024–1032. PMID: 20722676.
Article57. Fonseca VA, Alvarado-Ruiz R, Raccah D, Boka G, Miossec P, Gerich JE. EFC6018 GetGoal-Mono Study Investigators. Efficacy and safety of the once-daily GLP-1 receptor agonist lixisenatide in monotherapy: a randomized, double-blind, placebo-controlled trial in patients with type 2 diabetes (GetGoal-Mono). Diabetes Care. 2012; 35:1225–1231. PMID: 22432104.
Article58. Seino Y, Min KW, Niemoeller E, Takami A. EFC10887 GETGOAL-L Asia Study Investigators. Randomized, double-blind, placebo-controlled trial of the once-daily GLP-1 receptor agonist lixisenatide in Asian patients with type 2 diabetes insufficiently controlled on basal insulin with or without a sulfonylurea (GetGoal-L-Asia). Diabetes Obes Metab. 2012; 14:910–917. PMID: 22564709.59. Ryan GJ, Foster KT, Jobe LJ. Review of the therapeutic uses of liraglutide. Clin Ther. 2011; 33:793–811. PMID: 21741090.
Article60. Mori Y, Taniguchi Y, Sezaki K, Yokoyama J, Utsunomiya K. Liraglutide narrows the range of circadian glycemic variations in Japanese type 2 diabetes patients and nearly flattens these variations in drug-naive type 2 diabetes patients: a continuous glucose monitoring-based study. Diabetes Technol Ther. 2011; 13:1139–1144. PMID: 21877924.
Article61. Zinman B, Schmidt WE, Moses A, Lund N, Gough S. Achieving a clinically relevant composite outcome of an HbA1c of <7% without weight gain or hypoglycaemia in type 2 diabetes: a meta-analysis of the liraglutide clinical trial programme. Diabetes Obes Metab. 2012; 14:77–82. PMID: 21883806.62. Marre M, Shaw J, Brandle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, Le Thi TD, Colagiuri S. LEAD-1 SU study group. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009; 26:268–278. PMID: 19317822.63. Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, Ravn GM, Simo R. Liraglutide Effect and Action in Diabetes 5 (LEAD-5) met+SU Study Group. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009; 52:2046–2055. PMID: 19688338.
Article64. Parks M, Rosebraugh C. Weighing risks and benefits of liraglutide: the FDA's review of a new antidiabetic therapy. N Engl J Med. 2010; 362:774–777. PMID: 20164475.65. Bjerre Knudsen L, Madsen LW, Andersen S, Almholt K, de Boer AS, Drucker DJ, Gotfredsen C, Egerod FL, Hegelund AC, Jacobsen H, Jacobsen SD, Moses AC, Molck AM, Nielsen HS, Nowak J, Solberg H, Thi TD, Zdravkovic M, Moerch U. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology. 2010; 151:1473–1486. PMID: 20203154.
Article66. DeYoung MB, MacConell L, Sarin V, Trautmann M, Herbert P. Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes Technol Ther. 2011; 13:1145–1154. PMID: 21751887.
Article67. Kim D, MacConell L, Zhuang D, Kothare PA, Trautmann M, Fineman M, Taylor K. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007; 30:1487–1493. PMID: 17353504.
Article68. Madsbad S, Kielgast U, Asmar M, Deacon CF, Torekov SS, Holst JJ. An overview of once-weekly glucagon-like peptide-1 receptor agonists: available efficacy and safety data and perspectives for the future. Diabetes Obes Metab. 2011; 13:394–407. PMID: 21208359.69. Murphy CE. Review of the safety and efficacy of exenatide once weekly for the treatment of type 2 diabetes mellitus. Ann Pharmacother. 2012; 46:812–821. PMID: 22669803.
Article70. Macconell L, Pencek R, Li Y, Maggs D, Porter L. Exenatide once weekly: sustained improvement in glycemic control and cardiometabolic measures through 3 years. Diabetes Metab Syndr Obes. 2013; 6:31–41. PMID: 23358123.71. Rosenstock J, Reusch J, Bush M, Yang F, Stewart M. Albiglutide Study Group. Potential of albiglutide, a long-acting GLP-1 receptor agonist, in type 2 diabetes: a randomized controlled trial exploring weekly, biweekly, and monthly dosing. Diabetes Care. 2009; 32:1880–1886. PMID: 19592625.72. Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ 3rd. EGO Study Group. The effects of LY2189265, a long-acting glucagon-like peptide-1 analogue, in a randomized, placebo-controlled, double-blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab. 2011; 13:418–425. PMID: 21251180.
Article73. Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once-weekly GLP-1 analogue dulaglutide for 12 weeks in patients with type 2 diabetes: dose-dependent effects on glycaemic control in a randomized, double-blind, placebo-controlled study. Diabet Med. 2012; 29:1260–1267. PMID: 22804250.74. Watson E, Jonker DM, Jacobsen LV, Ingwersen SH. Population pharmacokinetics of liraglutide, a once-daily human glucagon-like peptide-1 analog, in healthy volunteers and subjects with type 2 diabetes, and comparison to twice-daily exenatide. J Clin Pharmacol. 2010; 50:886–894. PMID: 20133507.
Article75. Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011; 60:1561–1565. PMID: 21430088.
Article76. Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, Zychma M, Blonde L. LEAD-6 Study Group. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: a 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6). Lancet. 2009; 374:39–47. PMID: 19515413.
Article77. Buse JB, Sesti G, Schmidt WE, Montanya E, Chang CT, Xu Y, Blonde L, Rosenstock J. Liraglutide Effect Action in Diabetes-6 Study Group. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care. 2010; 33:1300–1303. PMID: 20332351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Global Shortage of Glucagon-Like Peptide-1 Receptor Agonists
- Clinical Application of Glucagon-Like Peptide-1 Receptor Agonists
- Glucagon-Like Peptide-1 Receptor Agonists for the Treatment of Type 2 Diabetes Mellitus: A Position Statement of the Korean Diabetes Association
- Glucagon-like peptide-1 and glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes
- New Potential Targets of Glucagon-Like Peptide 1 Receptor Agonists in Pancreatic β-Cells and Hepatocytes