Anat Cell Biol.
2011 Mar;44(1):35-40. 10.5115/acb.2011.44.1.35.
Characterization of the antigenic phenotype of alphaB-crystallin-expressing peripapillary glial cells in the developing chick retina
- Affiliations
-
- 1Department of Anatomy, School of Medicine, Chungbuk National University, Cheongju, Korea. seojh@chungbuk.ac.kr
- KMID: 2168866
- DOI: http://doi.org/10.5115/acb.2011.44.1.35
Abstract
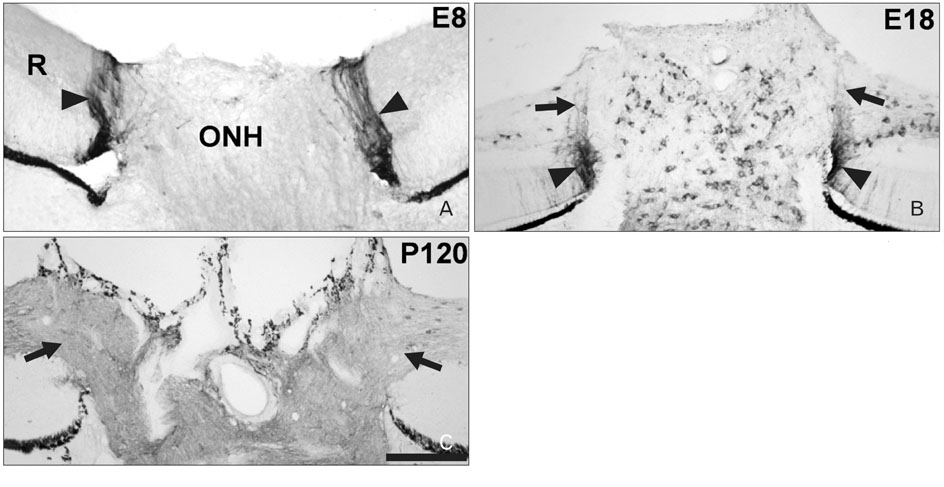
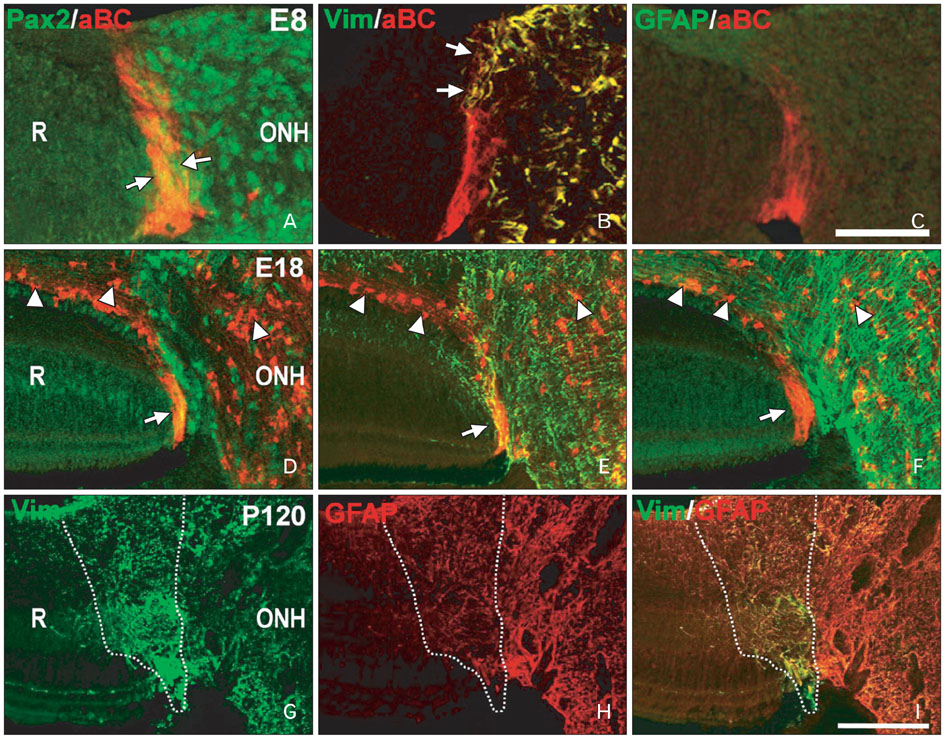
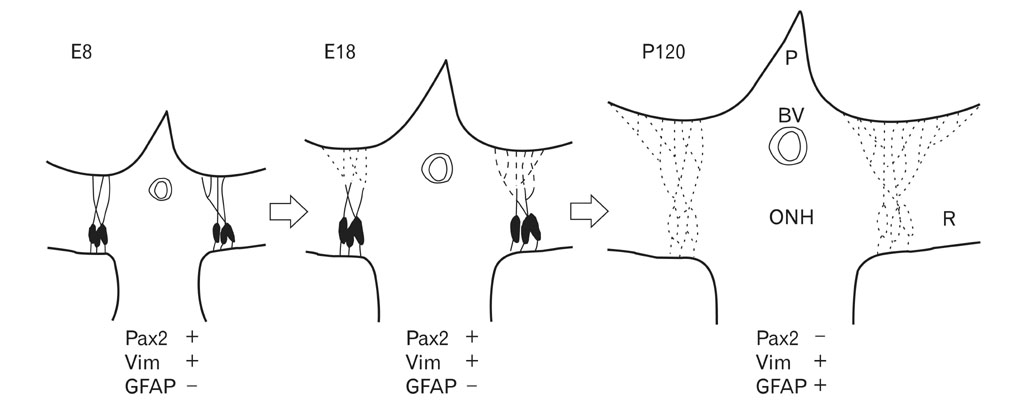
- Radial glia are transdifferentiated into astrocytes within the developing brain and spinal cord. The neural retina contains Muller cells, which are retinal radial glia. Some of the cells that surround the optic nerve head among Muller cells in the chicken retina are called peripapillary glial cells (PPGCs). PPGCs express different molecules compared to typical Muller cells. However, an antigenic PPGC phenotype has not yet been clearly established. In this study, we classified the antigenic PPGC phenotypes and identified the differentiation stages of these cells. At embryonic day (E)8, alphaB-crystallin-positive PPGCs had a bipolar shape with long processes that traversed entire layers of the retina. Pax2 and vimentin were expressed in alphaB-crystallin-positive PPGCs. Glial fibrillary acidic protein (GFAP) immunoreactivity was not observed in PPGCs. At E18, alphaB-crystallin immunoreactivity disappeared from the vitread processes of PPGCs. However, the PPGC cell bodies and ventricular processes contained alphaB-crystallin protein, and the PPGCs retained the same Pax2-positive/vimentin-positive/GFAP-negative profile as that seen at E8. At post-hatch day 120, alphaB-crystallin and Pax2 immunoreactivity was not observed, but vimentin and GFAP expression was clearly observed in the presumptive location of the PPGCs. Furthermore, these two proteins overlapped within that location. Considering that vimentin expression is prolonged until the post-hatching period in chicken brain, these findings suggest that Pax2-negative/vimentin-positive/GFAP-positive PPGCs are phenotypically identical to mature astrocytes in this avian species.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Metformin treatment confers protection of the optic nerve following photoreceptor degeneration
Sohair A. Eltony, Heba S. Mohaseb, Manal M. Sayed, Amel A. Ahmed
Anat Cell Biol. 2021;54(2):249-258. doi: 10.5115/acb.20.320.
Reference
-
1. Culican SM, Baumrind NL, Yamamoto M, Pearlman AL. Cortical radial glia: identification in tissue culture and evidence for their transformation to astrocytes. J Neurosci. 1990. 10:684–692.2. Gray GE, Sanes JR. Lineage of radial glia in the chicken optic tectum. Development. 1992. 114:271–283.3. Barry D, McDermott K. Differentiation of radial glia from radial precursor cells and transformation into astrocytes in the developing rat spinal cord. Glia. 2005. 50:187–197.4. Chan-Ling T, Chu Y, Baxter L, Weible Ii M, Hughes S. In vivo characterization of astrocyte precursor cells (APCs) and astrocytes in developing rat retinae: differentiation, proliferation, and apoptosis. Glia. 2009. 57:39–53.5. Gerhardt H, Schuck J, Wolburg H. Differentiation of a unique macroglial cell type in the pecten oculi of the chicken. Glia. 1999. 28:201–214.6. Schuck J, Gerhardt H, Wolburg H. The peripapillary glia of the optic nerve head in the chicken retina. Anat Rec. 2000. 259:263–275.7. Quesada A, Prada FA, Aguilera Y, Espinar A, Carmona A, Prada C. Peripapillary glial cells in the chick retina: a special glial cell type expressing astrocyte, radial glia, neuron, and oligodendrocyte markers throughout development. Glia. 2004. 46:346–355.8. Kim JY, Sohn HJ, Lee EY, Goo YS, Kim DW, Seo JH. Expression of alphaB-crystallin in the peripapillary glial cells of the developing chick retina. Neurochem Res. 2011. 36:76–82.9. Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992. 195:231–272.10. Linser PJ, Irvin CK. Immunohistochemical characterization of delta crystallin-containing retina/optic nerve "boundary" cells in the chick embryo. Dev Biol. 1987. 121:499–509.11. Klemenz R, Fröhli E, Steiger RH, Schäfer R, Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci U S A. 1991. 88:3652–3656.12. de Jong WW, Leunissen JA, Voorter CE. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993. 10:103–126.13. Lee DC, Kim RY, Wistow GJ. An avian alpha B-crystallin: non-lens expression and sequence similarities with both small (HSP27) and large (HSP70) heat shock proteins. J Mol Biol. 1993. 232:1221–1226.14. Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992. 89:10449–10453.15. Ganea E. Chaperone-like activity of alpha-crystallin and other small heat shock proteins. Curr Protein Pept Sci. 2001. 2:205–225.16. Soukkarieh C, Agius E, Soula C, Cochard P. Pax2 regulates neuronal-glial cell fate choice in the embryonic optic nerve. Dev Biol. 2007. 303:800–813.17. Mi H, Barres BA. Purification and characterization of astrocyte precursor cells in the developing rat optic nerve. J Neurosci. 1999. 19:1049–1061.18. Stanke J, Moose HE, El-Hodiri HM, Fischer AJ. Comparative study of Pax2 expression in glial cells in the retina and optic nerve of birds and mammals. J Comp Neurol. 2010. 518:2316–2333.19. Reichenbach A, Stolzenburg JU, Eberhardt W, Chao TI, Dettmer D, Hertz L. What do retinal muller (glial) cells do for their neuronal 'small siblings'? J Chem Neuroanat. 1993. 6:201–213.20. Rompani SB, Cepko CL. A common progenitor for retinal astrocytes and oligodendrocytes. J Neurosci. 2010. 30:4970–4980.21. Chu Y, Hughes S, Chan-Ling T. Differentiation and migration of astrocyte precursor cells and astrocytes in human fetal retina: relevance to optic nerve coloboma. FASEB J. 2001. 15:2013–2015.22. Dahl D, Crosby CJ, Sethi JS, Bignami A. Glial fibrillary acidic (GFA) protein in vertebrates: immunofluorescence and immunoblotting study with monoclonal and polyclonal antibodies. J Comp Neurol. 1985. 239:75–88.23. Kálmán M, Székely AD, Csillag A. Distribution of glial fibrillary acidic protein and vimentin-immunopositive elements in the developing chicken brain from hatch to adulthood. Anat Embryol (Berl). 1998. 198:213–235.24. Seo JH, Chang JH, Song SH, Lee HN, Jeon GS, Kim DW, Chung CK, Cho SS. Spatiotemporal gradient of astrocyte development in the chick optic tectum: evidence for multiple origins and migratory paths of astrocytes. Neurochem Res. 2008. 33:1346–1355.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of alphaB-crystallin mRNA using Single-stranded DNA Probe in Oligodendrocytes of the Developing Chick Retina
- Expression of Heat Shock Protein 27 and Alpha B Crystallin in the Retina and Optic Nerve of the Chick Embryo
- Expression of HSP27 and alphaB-crystallin in Avian Cerebellum during Development
- Induction of alphaB-crystallin in the hippocampus of KA-treated mouse brain
- alphaB-Crystallin is a Novel Oncoprotein Associated with Poor Prognosis in Breast Cancer