Cancer Res Treat.
2004 Dec;36(6):395-399.
Proteome Analysis of Differential Protein Expression in Cervical Cancer Cells after Paclitaxel Treatment
- Affiliations
-
- 1Department of Medical Bioscience, Graduate School of Catholic University, Korea. jspark@catholic.ac.kr
- 2Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Catholic University Medical College, Korea.
- 3Department of Bioscience and Biotechnology/Institute of Bioscience, Sejong University, Republic of Korea, Korea.
Abstract
- PURPOSE
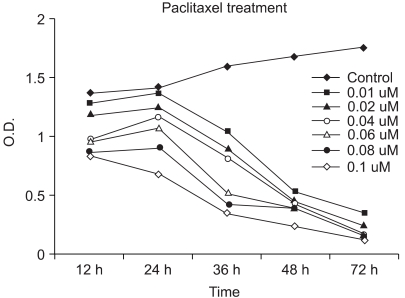
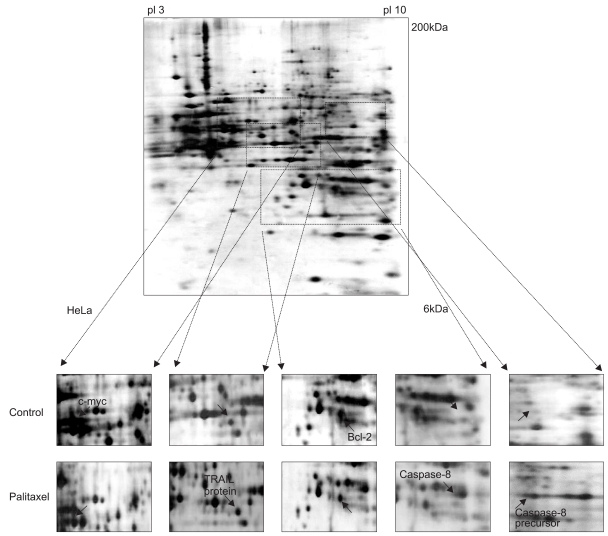
It is well known that infection with HPV (human papillomavirus) is the main cause of cervical cancer and certain types of HPV are recognized as carcinogens. At present, there is little information regarding the antineoplastic mechanism of paclitaxel against cervical carcinoma cells. We thus tried to analyze differential protein expression and antineoplastic mechanism-related proteins after paclitaxel treatment on cervical cancer cells by using a proteomic analysis and to investigate the mechanism of action. MATERIALS AND METHODS: Using proteomics analysis including 2-DE and MALDI-TOF-MS, we detected the antineoplastic mechanism-related proteins. Then, we performed western blot analysis for apoptosis- and transformation- related proteins to confirm expression patterns derived from proteome analysis after paclitaxel treatment. RESULTS: We identified several cellular proteins that are responsive to paclitaxel treatment in HeLa cells using proteomics methods. Paclitaxel treatment elevated main-ly apoptosis, immune response and cell cycle check point- related proteins. On the other hand, paclitaxel treatment diminished growth factor/oncogene-related proteins and transcription regulation-related proteins. Also, in the HPV-associated cervical carcinoma cells, paclitaxel demonstrated anti-proliferative activity through the membrane death receptor-mediated apoptotic pathway and the mitochondrial-mediated pathway. CONCLUSION: Identification and characterization of functionally modulated proteins involved in anti-cancer regulatory events should lead to a better nderstanding of the long-term actions of paclitaxel at the molecular level and will contribute to the future development of novel therapeutic drug treatments based upon current therapies.
Keyword
MeSH Terms
Figure
Reference
-
1. Vacca A, Ribatti D, Iurlaro M, Merchionne F, Nico B, Ria R, et al. Docetaxel versus paclitaxel for antiangiogenesis. J Hematother Stem Cell Res. 2002; 11:103–118. PMID: 11847007.
Article2. Hotchkiss KA, Ashton AW, Mahmood R, Russell RG, Sparano JA, Schwartz EL. Inhibition of endothelial cell function in vitro and angiogenesis in vivo by docetaxel (Taxotere): association with impaired repositioning of the microtubule organizing center. Mol Cancer Ther. 2002; 1:1191–1200. PMID: 12479700.3. Belani C, Lynch T. Docetaxel (Taxotere) in combination with platinums in patients with non-small cell lung cancer: trial data and implications for clinical management. Semin Oncol. 2001; 28(Suppl 2):10–14.
Article4. Burris HA 3rd. Docetaxel (Taxotere) plus trastuzumab (Herceptin) in breast cancer. Semin Oncol. 2001; 28:38–44. PMID: 11301373.
Article5. Hortobagyi GN. Recent progress in the clinical development of docetaxel (Taxotere). Semin Oncol. 1999; 26:32–36. PMID: 10426457.6. Rose WC, Fairchild C, Lee FY. Preclinical antitumor activity of two novel taxanes. Cancer Chemother Pharmacol. 2001; 47:97–105. PMID: 11269747.
Article7. Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc. 1971; 93:2325–2327. PMID: 5553076.8. Kim JH, Chin BR, Kim SY, Kim JR, Baek SH. Differential sensitivity of Taxol-induced Apoptosis in U2OS and SaOS2 Osteogenic Sarcoma Cells. Cancer Res Treat. 2003; 35:148–153.
Article9. Ferlini C, Raspaglio G, Mozzetti S, Distefano M, Filippetti F, Martinelli E, et al. Bcl-2 down-regulation is a novel mechanism of paclitaxel resistance. Mol Pharmacol. 2003; 64:51–58. PMID: 12815160.
Article10. Krug LM, Miller VA, Filippa DA, Venkatraman E, Ng KK, Kris MG. Bcl-2 and bax expression in advanced non-small cell lung cancer: lack of correlation with chemotherapy response or survival in patients treated with docetaxel plus vinorelbine. Lung Cancer. 2003; 39:139–143. PMID: 12581565.
Article11. Chun E, Lee KY. Bcl-2 and Bcl-xL are important for the induction of paclitaxel resistance in human hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2004; 315:771–779. PMID: 14975768.
Article12. Heliez C, Baricault L, Barboule N, Valette A. Paclitaxel increases p21 synthesis and accumulation of its AKT-phosphorylated form in the cytoplasm of cancer cells. Oncogene. 2003; 22:3260–3268. PMID: 12761496.
Article13. Tudor G, Aguilera A, Halverson DO, Laing ND, Sausville EA. Susceptibility to drug-induced apoptosis correlates with differential modulation of Bad, Bcl-2 and Bcl-xL protein levels. Cell Death Differ. 2000; 7:574–586. PMID: 10822281.
Article14. Wang J, Kobayashi M, Han M, Choi S, Takano M, Hashino S, et al. MyD88 is involved in the signalling pathway for Taxol-induced apoptosis and TNF-alpha expression in human myelomonocytic cells. Br J Haematol. 2002; 118:638–645. PMID: 12139759.15. Eric KR. Update on the antitumor activity of paclitaxel in clinical trials. Ann Pharmacother. 1994; 28:S18–S22. PMID: 7915154.
Article16. Holmes FA, Kudelka AP, Kavanagh JJ. Georg GI, editor. Taxane Antineoplastic Agents. 1995. Washington DC: ACS;p. 31–57.17. Rowinsky EK, Cazenave LA, Donehower RC. Taxol-a novel investigational antimicrotubule agent. J Natl Cancer Inst. 1990; 82:1247–1259. PMID: 1973737.18. Crossin KL, Carney DH. Microtubule stabilization by taxol inhibits initiation of DNA synthesis by thrombin and epidermal growth factor. Cell. 1981; 27:341–350. PMID: 6120766.19. Wilson L, Panda D, Jordan MA. Modulation of microtubule dynamics by drugs: a paradigm for the actions of cellular regulators. Cell Struct Funct. 1999; 24:329–335. PMID: 15216890.
Article20. Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA. 1993; 90:9552–9556. PMID: 8105478.
Article21. Ling X, Bernacki RJ, Brattain MG, Li F. Induction of survivin expression by taxol (paclitaxel) is an early event, which is independent of taxol-mediated G2/M arrest. J Biol Chem. 2004; 279:15196–15203. PMID: 14722122.
Article22. Chen ST, Pan TL, Tsai YC, Huang CM. Proteomics reveals protein profile changes in doxorubicin-treated MCF-7 human breast cancer cells. Cancer Lett. 2002; 181:95–107. PMID: 12430184.23. Pusztai L, Gregory BW, Baggerly KA, Peng B, Koomen J, Kuerer HM, et al. Pharmacoproteomic analysis of prechemotherapy and postchemotherapy plasma samples from patients receiving neoadjuvant or adjuvant chemotherapy for breast carcinoma. Cancer. 2004; 100:1814–1822. PMID: 15112261.
Article24. MacKeigan JP, Clements CM, Lich JD, Pope RM, Hod Y, Ting JP. Proteomic profiling drug-induced apoptosis in non-small cell lung carcinoma: identification of RS/DJ-1 and RhoGDIalpha. Cancer Res. 2003; 63:6928–6934. PMID: 14583493.25. Mian S, Ball G, Hornbuckle J, Holding F, Carmichael J, Ellis I, et al. A prototype methodology combining surface-enhanced laser desorption/ionization protein chip technology and artificial neural network algorithms to predict the chemoresponsiveness of breast cancer cell lines exposed to Paclitaxel and Doxorubicin under in vitro conditions. Proteomics. 2003; 3:1725–1737. PMID: 12973733.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Identification of TACC3 (Transforming Acidic Coiled-Coil 3) as a novel target of paclitaxel-mediated tumor therapy in cervical cancer cells
- Quantitative analysis of proteins related to chemoresistance to paclitaxel and carboplatin in human SiHa cervical cancer cells via iTRAQ
- Clusterin expression and paclitaxel resistance in cervical cancer cell lines
- Blockade of Autophagy Aggravates Endoplasmic Reticulum Stress and Improves Paclitaxel Cytotoxicity in Human Cervical Cancer Cells
- Mechanism of FHIT-Induced Apoptosis in Lung Cancer Cell Lines