Cancer Res Treat.
2004 Dec;36(6):360-366.
Responsiveness of CPT-11 in Respect to hMLH1 and hMSH2 Protein Expression in the Primary Colorectal Cancer
- Affiliations
-
- 1Department of Surgery, University of Ulsan College of Medicine, Korea. jckim@amc. seoul.kr
- 2Colorectal Cancer Team, Asan Medical Center and Asan Cancer Center, Seoul, Korea.
Abstract
- PURPOSE
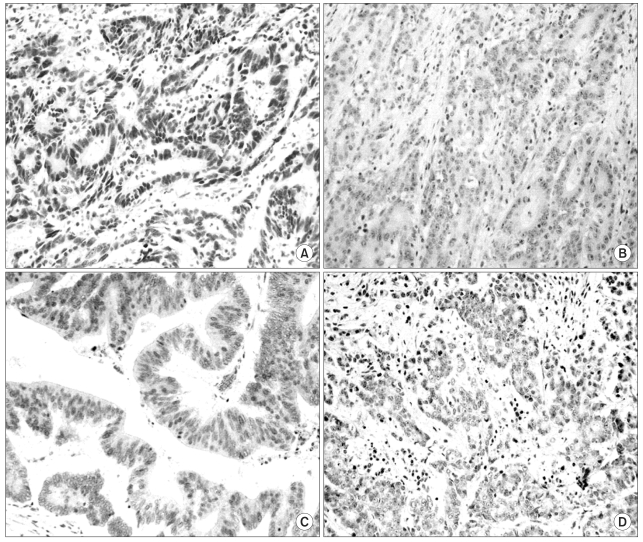
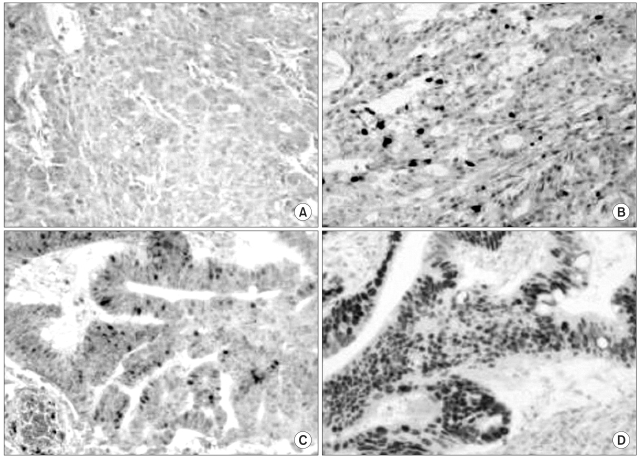
The aim of this study was to evaluate the responsiveness to CPT-11 with respect to hMLH1 and hMSH2 protein expressions in primary colorectal tumors. MATERIAL AND METHODS: 91 patients with colorectal cancer treated having undergone surgery and postoperative CPT-11-based adjuvant chemotherapy, between 1997 and 2002, were prospectively recruited. Tumor samples were immunohistochemically analyzed for the expressions of hMLH1, hMSH2, p53 and CEA proteins. RESULTS: Of the 91 tumors, 6 (6.6%) and 4 (4.4%) showed loss of hMLH1 and hMSH2 protein expressions, respectively. The response rate of patients with tumors not expressing either hMLH1 or hMSH2 was higher than that of those expressing either of these proteins (p=0.026). Patients with tumors not expressing hMLH1 showed a significantly better response to CPT-11 (p=0.04). The responsiveness was not associated with the expressions of hMSH2, p53 or CEA. There were no correlations between drug toxicity and the expressions of hMLH1, hMSH2 or p53. The overall survival was better in patients responsive to CPT-11-based chemotherapy compared to non-responders. CONCLUSION: The immunohistochemical determination of loss of hMLH1 and hMSH2 expressions may be used in determining the responsiveness to CPT-11-based chemotherapy. Our results suggest that hMLH1 protein expression may be a predictor for CPT-11 responsiveness in patients with colorectal cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Pisani P, Parkin DM, Ferlay J. Estimates of the worldwide mortality from eighteen major cancers in 1985. Implications for prevention and projections of future burden. Int J Cancer. 1993; 55:891–903. PMID: 8253525.
Article2. Cunningham D, Findlay M. The chemotherapy of colon cancer can no longer be ignored. Eur J Cancer. 1993; 29A:2077–2079. PMID: 8297642.
Article3. Cunningham D. Current status of colorectal cancer: CPT-11 (irinotecan), a therapeutic innovation. Eur J Cancer. 1996; 32(suppl):S1–S8. PMID: 8943658.
Article4. Conti JA, Kemeny NE, Saltz LB, Huang Y, Tong WP, Chou TC, et al. Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol. 1996; 14:709–715. PMID: 8622015.
Article5. Vanhoefer U, Harstrick A, Achterrath W, Cao S, Seeber S, Rustum YM. Irinotecan in the treatment of colorectal cancer: clinical overview. J Clin Oncol. 2001; 19:1501–1518. PMID: 11230497.6. Won YW, Lim YH, Park HY, Oh HS, Choi JH, Lee YY, et al. Phase II study of irinotecan, 5-fluorouracil, and leucovorin in relapsed or metastatic colorectal cancer as first-line therapy. Cancer Res Treat. 2004; 36:235–239.
Article7. Jiricny J, Nystrom-Lahti M. Mismatch repair defects in cancer. Curr Opin Genet Dev. 2000; 10:157–161. PMID: 10753784.
Article8. Claij N, te Riele H. Microsatellite instability in human cancer: a prognostic marker for chemotherapy? Exp Cell Res. 1999; 246:1–10. PMID: 9882509.
Article9. Kim JC, Han MS, Lee HK, Kim WS, Park SC, Bodmer WF, et al. Distribution of carcinoembryonic antigen and biologic behavior in colorectal carcinoma. Dis Colon Rectum. 1999; 42:640–648. PMID: 10344687.
Article10. WHO offset publication No. 48. WHO handbook for reporting results of cancer treatment. 1979. Geneva: WHO.11. NCI-NIH National Cancer Institute. version 2.0. 1999. Bethesda, MD: NCI-NIH;p. 1–80.12. Gryfe R, Kim H, Hsieh ET, Aronson MD, Holowaty EJ, Bull SB, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000; 342:69–77. PMID: 10631274.
Article13. Ward R, Meagher A, Tomlinson I, O'Connor T, Norrie M, Wu R, et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut. 2001; 48:821–829. PMID: 11358903.
Article14. Buyse M, Piedbois Y, Piedbois P, Gray R. Tumor site, sex, and survival in colorectal cancer. Lancet. 2000; 356:858. PMID: 11022959.15. Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumor site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000; 355:1745–1750. PMID: 10832824.16. Branch P, Aquilina G, Bignami M, Karran P. Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature. 1993; 15:652–654. PMID: 8464518.
Article17. Fink D, Nebel S, Aebi S, Zheng H, Cenni B, Nehme A, et al. The role of DNA mismatch repair in platinum drug resistance. Cancer Res. 1996; 56:4881–4886. PMID: 8895738.18. Bras-Goncalves RA, Rosty C, Laurent-Puig P, Soulie P, Dutrillaux B, Poupon MF. Sensitivity to CPT-11 of xenografted human colorectal cancers as a function of microsatellite instability and p53 status. Br J Cancer. 2000; 82:913–923. PMID: 10732766.
Article19. Fallik D, Borrini F, Boige V, Viguier J, Jacob S, Miquel C, et al. Microsatellite instability is a predictive factor of the tumor response to irinotecan in patients with advanced colorectal cancer. Cancer Res. 2003; 63:5738–5744. PMID: 14522894.20. Marcus VA, Madlensky L, Gryfe R, Kim H, So K, Millar A, et al. Immunohistochemistry for hMLH1 and hMSH2: a practical test for DNA mismatch repair-deficient tumors. Am J Surg Pathol. 1999; 23:1248–1255. PMID: 10524526.
Article21. Stone JG, Robertson D, Houlston RS. Immunohistochemirstry for MSH2 and MLH1: a method for identifying mismatch repair deficient colorectal cancer. J Clin Pathol. 2001; 54:484–487. PMID: 11376026.22. Kim HC, Kim CN, Yu CS, Roh SA, Kim JC. Methylation of the hMLH1 and hMSH2 promoter in early-onset sporadic colorectal carcinomas with microsatellite instability. Int J Colorectal Dis. 2003; 18:196–202. PMID: 12673483.
Article23. Creemers GJ, Lund B, Verweij J. Topoisomerase I inhibitors: topotecan and irinotecan. Cancer Treat Rev. 1994; 20:73–96. PMID: 8293429.24. Elliott B, Jasin M. Repair of double-strand breaks by homologous recombination in mismatch repair-defective mammalian cells. Mol Cell Biol. 2001; 21:2671–2682. PMID: 11283247.
Article25. Petersen S, Thames HD, Nieder C, Petersen C, Baumann M. The results of colorectal cancer treatment by p53 status: treatment-specific overview. Dis Colon Rectum. 2001; 44:322–333. PMID: 11289276.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of hMSH2, hMLH1 Protein in Sporadic Colorectal Cancer and Corresponding Normal Tissue
- hMLH1/hMSH2 Protein Expression in Sporadic Colorectal Carcinoma and Its Clinicopathological Significance
- Clinicopathological Correlation of hMLH1 and hMSH2 Protein Expressions in Stage III Colon Cancer
- Characterization of Mutator Pathway in Younger-age-onset Colorectal Adenocarcinomas
- Efficacy of hMLH1/hMSH2 Immunohistochemical Staining as Representative Index for Microsatellite Instability Status in Sporadic Colorectal Cancer