Cancer Res Treat.
2005 Dec;37(6):332-338.
Randomized, Multi-center Phase II Trial of Docetaxel Plus Cisplatin Versus Etoposide Plus Cisplatin as the First-line Therapy for Patients with Advanced Non-Small Cell Lung Cancer
- Affiliations
-
- 1Department of Internal Medicine, College of Medicine, Soon Chun Hyang University, Seoul, Korea. parkhs@hosp.sch.ac.kr
- 2Department of Internal Medicine, College of Medicine, Chungang University, Seoul, Korea.
- 3Department of Internal Medicine, College of Medicine, Yonsei University, Seoul, Korea.
- 4Department of Internal Medicine, College of Medicine, Hanyang University, Seoul, Korea.
- 5Department of Internal Medicine, College of Medicine, Hallym University, Seoul, Korea.
- 6Department of Internal Medicine, College of Medicine, Chonbuk National University, Jeonju, Korea.
- 7Department of Internal Medicine, College of Medicine, Inha University, Incheon, Korea.
- 8Aventis Pharma Korea, Seoul, Korea.
Abstract
- PURPOSE
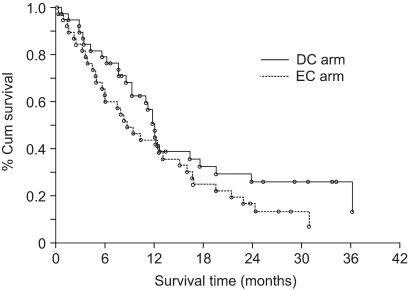
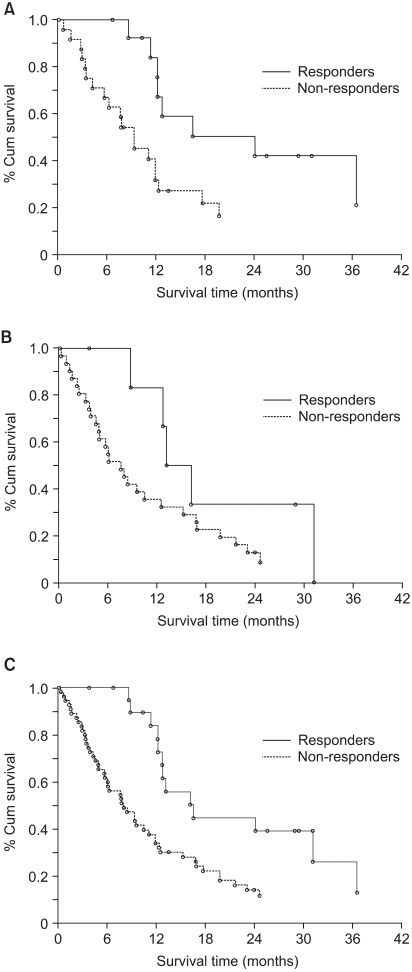
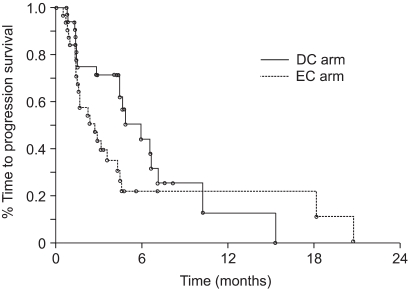
We prospectively conducted a multi-center, open-label, randomized phase II trial to compare the efficacy and safety of docetaxel plus cisplatin (DC) and etoposide plus cisplatin (EC) for treating advanced stage non-small cell lung cancer (NSCLC). MATERIALS AND METHODS: Seventy-eight previously untreated patients with locally advanced, recurrent or metastatic NSCLC were enrolled in this study. The patients received cisplatin 75 mg/m2 on day 1 and either docetaxel 75 mg/m2 on day 1 or etoposide 100 mg/m2 on days 1 to 3 in the DC or EC arm, respectively, every 3 weeks. RESULTS: The objective response rate was 39.4% (15/38) and 18.4% (7/38) (p=0.023) in the DC and EC arms, respectively. The median time to progression (TTP) was 5.9 and 2.7 months (p=0.119), and the overall survival was 12.1 and 8.7 months (p=0.168) in the DC and EC arms, respectively. The prognostic factors for longer survival were an earlier disease stage (stage III, p=0.0095), the responders to DC (p=0.0174) and the adenocarcinoma histology (p=0.0454). The grades 3 and 4 toxicities were similar in both arms, with more febrile neutropenia (7.9% vs. 0%) and fatigue (7.9% vs. 0%) being noted in the DC arm. CONCLUSION: DC offered a superior overall response rate than does EC, along with tolerable toxicity profiles, although the DC drug combination did not show significantly improved survival and TTP.
MeSH Terms
Figure
Reference
-
1. Vokes EE, Bitran JD, Vogelzang NJ. Chemotherapy for non-small cell lung cancer. The continuing challenge. Chest. 1991; 99:1326–1328. PMID: 1645242.2. De Vita VTJ, Hellman S, Rosenberg SA. Cancer: principles and practice of oncology. 2001. 6th ed. Philadelphia: J.B. Lippincott Co.;p. 925–983.3. Bunn PA Jr, Kelly K. New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: a review of the literature and future directions. Clin Cancer Res. 1998; 4:1087–1100. PMID: 9607565.4. Marino P, Pampallona S, Preatoni A, Cantoni A, Invernizzi F. Chemotherapy vs supportive care in advanced non-small-cell lung cancer. Results of a meta-analysis of the literature. Chest. 1994; 106:861–865. PMID: 7521815.5. Non-Small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomized clinical trials. BMJ. 1995; 311:899–909. PMID: 7580546.6. Grilli R, Oxman AD, Julian JA. Chemotherapy for advanced non-small-cell lung cancer: how much benefit is enough? J Clin Oncol. 1993; 11:1866–1872. PMID: 8410111.
Article7. Cullen MH, Billingham LJ, Woodroffe CM, Chetiyawardana AD, Gower NH, Joshi R, et al. Mitomycin, ifosfamide, and cisplatin in unresectable non-small-cell lung cancer: effects on survival and quality of life. J Clin Oncol. 1999; 17:3188–3194. PMID: 10506617.
Article9. Bunn PA Jr. The treatment of non-small cell lung cancer: current perspectives and controversies, future directions. Semin Oncol. 1994; 21(Suppl 6):49–59. PMID: 8052874.10. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, et al. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000; 18:2095–2103. PMID: 10811675.
Article11. Fossella FV, Lynch T, Shepherd FA. Second line chemotherapy for NSCLC: establishing a gold standard. Lung Cancer. 2002; 38(Suppl 4):5–12. PMID: 12480189.
Article12. Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, et al. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004; 22:330–353. PMID: 14691125.
Article13. Zalcberg J, Millward M, Bishop J, McKeage M, Zimet A, Toner G, et al. Phase II study of docetaxel and cisplatin in advanced non-small-cell lung cancer. J Clin Oncol. 1998; 16:1948–1953. PMID: 9586914.
Article14. Georgoulias V, Androulakis N, Dimopoulos AM, Kouroussis C, kakolyris S, Papadakis M, et al. First-line treatment of advanced non-small-cell lung cancer with docetaxel and cisplatin: a multicenter phase II study. Ann Oncol. 1998; 9:331–334. PMID: 9602269.
Article15. Fossella F, Pereira JR, von Pawel J, Pluzanska A, Gorbounova V, Kaukel E, et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non-small-cell lung cancer: the TAX 326 Study Group. J Clin Oncol. 2003; 21:3016–3024. PMID: 12837811.
Article16. Klastersky J. Therapy with cisplatin and etoposide for non-small-cell lung cancer. Semin Oncol. 1986; 13(Suppl 3):104–114. PMID: 3020693.17. Bunn PA Jr. The expanding role of cisplatin in the treatment of non-small-cell lung cancer. Semin Oncol. 1989; 16(Suppl 6):10–21. PMID: 2548280.18. Chung HG, Choi JH, Chung YS, Kim DL, Lee YS, Chang J, et al. Phase II trial of sequential VP-16, cisplatin combination chemotherapy and radiotherapy for locally advanced (stage III) non-small cell lung cancer. J Korean Cancer Assoc. 1991; 23:131–139.19. Kim KH, Oh SY, Jeong HS, Lee JT, Kim WS, Kim HJ, et al. Prolonged oral etoposide in combination with intravenous cisplatin for advanced non-small cell lung cancer. J Korean Cancer Assoc. 1999; 31:105–111.20. Paek CW, Yoon SY, Seo JH, Choi CW, Kim BS, Shin SW, et al. Concurrent chemoradiation therapy with cisplatin and oral etoposide for locally advanced non-small cell lung cancer. J Korean Cancer Assoc. 2000; 32:682–689.21. Akehurst RL, Beinert T, Crawford J, Crino L, Debus J, Edcersberger F, et al. Consensus on medical treatment of non-small cell lung cancer. Lung Cancer. 2002; 38(Suppl 3):S3–S13.
Article22. Kubota K, Watanabe K, Kunitoh H, Noda K, Ichinose Y, Katakami N, et al. Phase III randomized trial of docetaxel plus cisplatin versus vindesine plus cisplatin in patients with stage IV non-small-cell lung cancer: the Japanese Taxotere Lung Cancer Study Group. J Clin Oncol. 2004; 22:254–261. PMID: 14722033.
Article23. Kelly K, Crowley J, Bunn PA Jr, Presant CA, Grevstad PK, Moinpour CM, et al. Randomized phase III trial of paclitaxel plus carboplatin versus vinorelbine plus cisplatin in the treatment of patients with advanced non--small-cell lung cancer: a Southwest Oncology Group trial. J Clin Oncol. 2001; 19:3210–3218. PMID: 11432888.
Article24. Belani CP, Lee JS, Socinski MA, Robert F, Waterhouse D, Rowland K, et al. Randomized phase III trial comparing cis platin-etoposide to carboplatin-paclitaxel in advanced or metastatic non-small cell lung cancer. Ann Oncol. 2005; 16:1069–1075. PMID: 15860487.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A prospective randomized study of cisplatin versus PEV(cisplatin, etoposide, vinblastine) chemotherapy in advanced non-small cell lung cancer
- Treatment of Small Cell Lung Cancer
- Phase II trial of 5-FU, etoposide, cisplatin (FEP) combination chemotherapy in unresectable non-small cell lung cancer
- Combination chemotherapy with docetaxel and cisplatin as first-line treatment in advanced gastric cancer: is it a new effective chemotherapy?
- Phase II trial of sequential VP-16, cisplatin combination chemotherapy and radiotherapy for locally advanced (stage III) non-small cell lung cancer