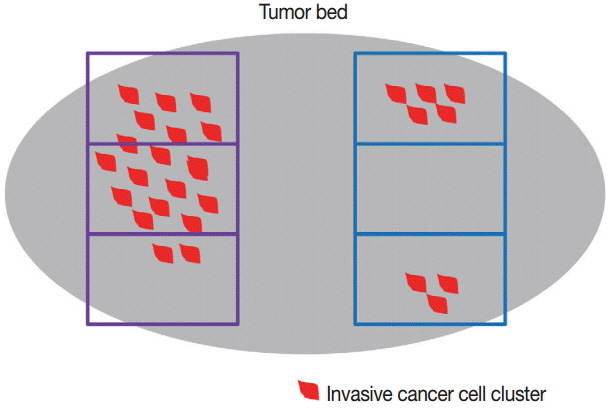
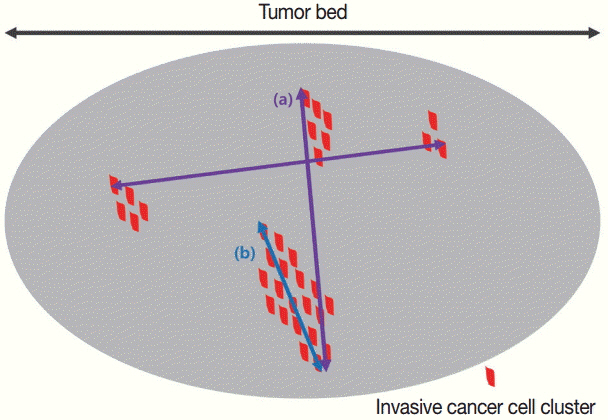
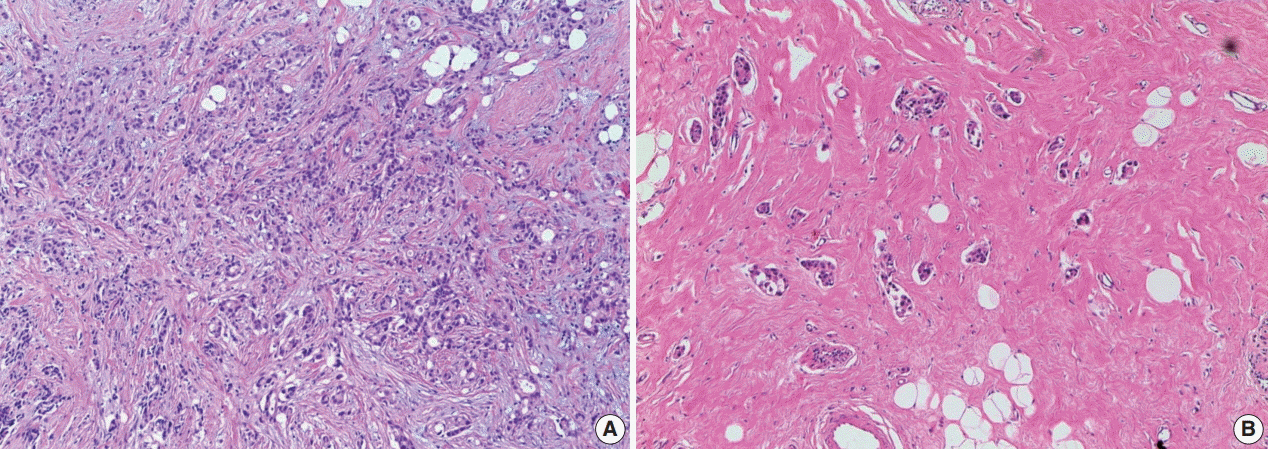
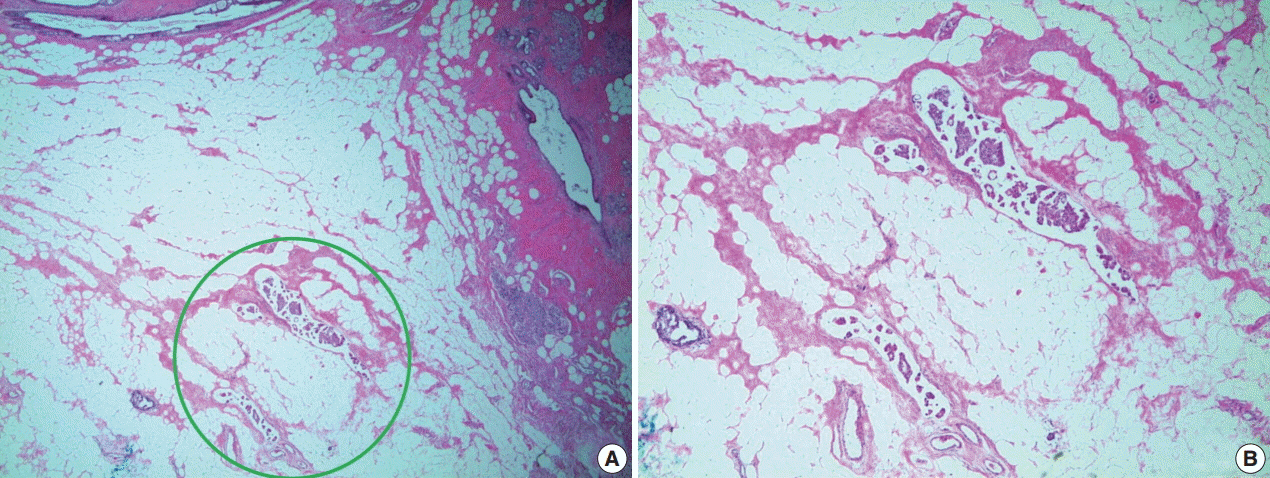
J Pathol Transl Med.
2016 May;50(3):173-180. 10.4132/jptm.2016.02.02.
Pathologic Evaluation of Breast Cancer after Neoadjuvant Therapy
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea. kjs1976@yuhs.ac
- KMID: 2164593
- DOI: http://doi.org/10.4132/jptm.2016.02.02
Abstract
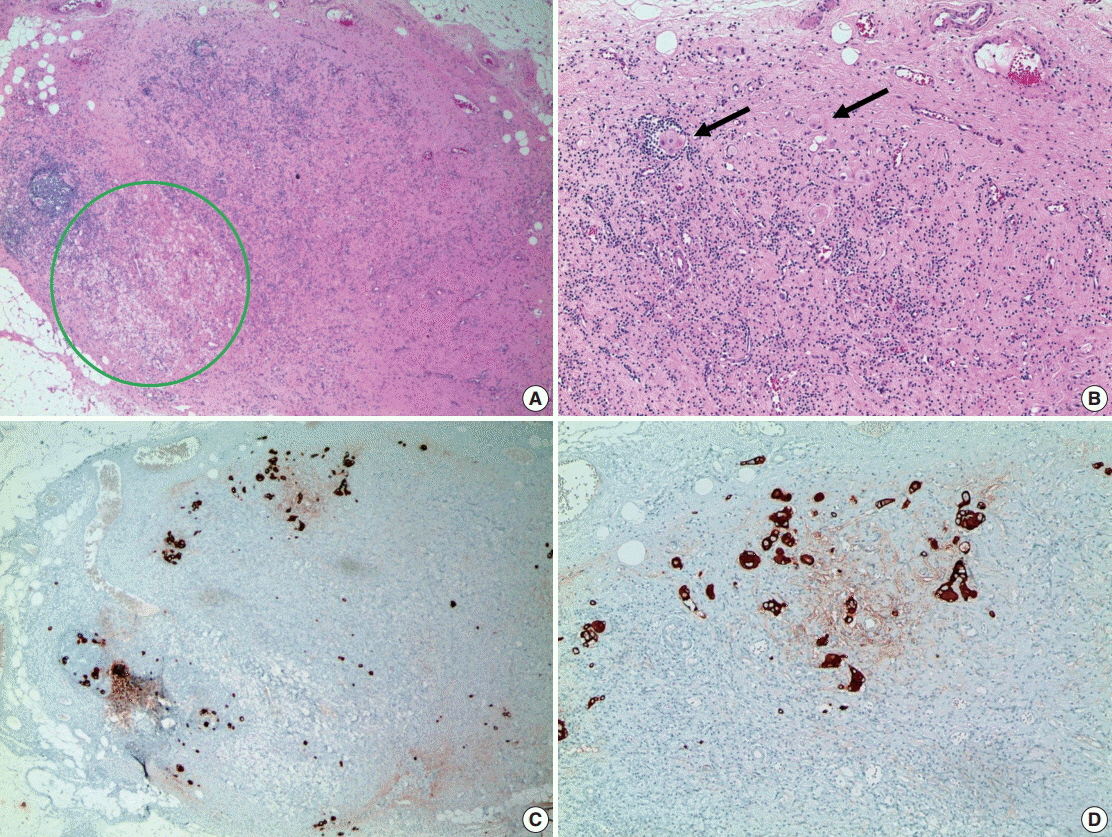
- Breast cancer, one of the most common cancers in women, has various treatment modalities. Neoadjuvant therapy (NAT) has been used in many clinical trials because it is easy to evaluate the treatment response to therapeutic agents in a short time period; consequently, NAT is currently a standard treatment modality for large-sized and locally advanced breast cancers, and its use in early-stage breast cancer is becoming more common. Thus, chances to encounter breast tissue from patients treated with NAT is increasing. However, systems for handling and evaluating such specimens have not been established. Several evaluation systems emphasize a multidisciplinary approach to increase the accuracy of breast cancer assessment. Thus, detailed and systematic evaluation of clinical, radiologic, and pathologic findings is important. In this review, we compare the major problems of each evaluation system and discuss important points for handling and evaluating NAT-treated breast specimens.
Figure
Cited by 2 articles
-
The Role of Neutrophil-lymphocyte Ratio and Platelet-lymphocyte Ratio in Predicting Neoadjuvant Chemotherapy Response in Breast Cancer
Hee Yeon Kim, Tae Hyun Kim, Hye Kyoung Yoon, Anbok Lee
J Breast Cancer. 2019;22(3):425-438. doi: 10.4048/jbc.2019.22.e41.Early Prediction of Response to Neoadjuvant Chemotherapy Using Dynamic Contrast-Enhanced MRI and Ultrasound in Breast Cancer
Yunju Kim, Sung Hun Kim, Byung Joo Song, Bong Joo Kang, Kwang-il Yim, Ahwon Lee, Yoonho Nam
Korean J Radiol. 2018;19(4):682-691. doi: 10.3348/kjr.2018.19.4.682.
Reference
-
1. Thompson AM, Moulder-Thompson SL. Neoadjuvant treatment of breast cancer. Ann Oncol. 2012; 23 Suppl 10:x231–6.
Article2. Moreno-Aspitia A. Neoadjuvant therapy in early-stage breast cancer. Crit Rev Oncol Hematol. 2012; 82:187–99.
Article3. Pierga JY, Mouret E, Laurence V, et al. Prognostic factors for survival after neoadjuvant chemotherapy in operable breast cancer: the role of clinical response. Eur J Cancer. 2003; 39:1089–96.4. Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999; 17:460–9.
Article5. Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998; 16:2672–85.
Article6. Esserman LJ, Woodcock J. Accelerating identification and regulatory approval of investigational cancer drugs. JAMA. 2011; 306:2608–9.
Article7. Bonadonna G, Veronesi U, Brambilla C, et al. Primary chemotherapy to avoid mastectomy in tumors with diameters of three centimeters or more. J Natl Cancer Inst. 1990; 82:1539–45.
Article8. Boughey JC, Peintinger F, Meric-Bernstam F, et al. Impact of preoperative versus postoperative chemotherapy on the extent and number of surgical procedures in patients treated in randomized clinical trials for breast cancer. Ann Surg. 2006; 244:464–70.
Article9. Mamounas EP, Anderson SJ, Dignam JJ, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012; 30:3960–6.
Article10. Carey LA, Metzger R, Dees EC, et al. American Joint Committee on Cancer tumor-node-metastasis stage after neoadjuvant chemotherapy and breast cancer outcome. J Natl Cancer Inst. 2005; 97:1137–42.
Article11. Ogston KN, Miller ID, Payne S, et al. A new histological grading system to assess response of breast cancers to primary chemotherapy: prognostic significance and survival. Breast. 2003; 12:320–7.
Article12. Abrial SC, Penault-Llorca F, Delva R, et al. High prognostic significance of residual disease after neoadjuvant chemotherapy: a retrospective study in 710 patients with operable breast cancer. Breast Cancer Res Treat. 2005; 94:255–63.
Article13. Pinder SE, Provenzano E, Earl H, Ellis IO. Laboratory handling and histology reporting of breast specimens from patients who have received neoadjuvant chemotherapy. Histopathology. 2007; 50:409–17.
Article14. Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007; 25:4414–22.
Article15. Dash N, Chafin SH, Johnson RR, Contractor FM. Usefulness of tissue marker clips in patients undergoing neoadjuvant chemotherapy for breast cancer. AJR Am J Roentgenol. 1999; 173:911–7.
Article16. Oh JL, Nguyen G, Whitman GJ, et al. Placement of radiopaque clips for tumor localization in patients undergoing neoadjuvant chemotherapy and breast conservation therapy. Cancer. 2007; 110:2420–7.
Article17. Schulz-Wendtland R, Heywang-Köbrunner SH, Aichinger U, Krämer S, Wenkel E, Bautz W. Do tissue marker clips after sonographically or stereotactically guided breast biopsy improve follow-up of small breast lesions and localisation of breast cancer after chemotherapy? Rofo. 2002; 174:620–4.18. Youn I, Choi SH, Kook SH, et al. Ultrasonography-guided surgical clip placement for tumor localization in patients undergoing neoadjuvant chemotherapy for breast cancer. J Breast Cancer. 2015; 18:44–9.
Article19. Margolin FR, Kaufman L, Denny SR, Jacobs RP, Schrumpf JD. Metallic marker placement after stereotactic core biopsy of breast calcifications: comparison of two clips and deployment techniques. AJR Am J Roentgenol. 2003; 181:1685–90.20. Provenzano E, Bossuyt V, Viale G, et al. Standardization of pathologic evaluation and reporting of postneoadjuvant specimens in clinical trials of breast cancer: recommendations from an international working group. Mod Pathol. 2015; 28:1185–201.
Article21. Bossuyt V, Provenzano E, Symmans WF, et al. Recommendations for standardized pathological characterization of residual disease for neoadjuvant clinical trials of breast cancer by the BIG-NABCG collaboration. Ann Oncol. 2015; 26:1280–91.
Article22. Sharkey FE, Addington SL, Fowler LJ, Page CP, Cruz AB. Effects of preoperative chemotherapy on the morphology of resectable breast carcinoma. Mod Pathol. 1996; 9:893–900.23. Honkoop AH, Pinedo HM, De Jong JS, et al. Effects of chemotherapy on pathologic and biologic characteristics of locally advanced breast cancer. Am J Clin Pathol. 1997; 107:211–8.
Article24. Diaz J, Stead L, Shapiro N, et al. Mitotic counts in breast cancer after neoadjuvant systemic chemotherapy and development of metastatic disease. Breast Cancer Res Treat. 2013; 138:91–7.
Article25. Rajan R, Poniecka A, Smith TL, et al. Change in tumor cellularity of breast carcinoma after neoadjuvant chemotherapy as a variable in the pathologic assessment of response. Cancer. 2004; 100:1365–73.
Article26. Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer. 2002; 95:681–95.27. Leitner SP, Swern AS, Weinberger D, Duncan LJ, Hutter RV. Predictors of recurrence for patients with small (one centimeter or less) localized breast cancer (T1a,b N0 M0). Cancer. 1995; 76:2266–74.28. Lee AK, Loda M, Mackarem G, et al. Lymph node negative invasive breast carcinoma 1 centimeter or less in size (T1a,bNOMO): clinicopathologic features and outcome. Cancer. 1997; 79:761–71.29. Rabban JT, Glidden D, Kwan ML, Chen YY. Pure and predominantly pure intralymphatic breast carcinoma after neoadjuvant chemotherapy: an unusual and adverse pattern of residual disease. Am J Surg Pathol. 2009; 33:256–63.30. Colleoni M, Rotmensz N, Maisonneuve P, et al. Prognostic role of the extent of peritumoral vascular invasion in operable breast cancer. Ann Oncol. 2007; 18:1632–40.
Article31. Sahoo S, Lester SC. Pathology of breast carcinomas after neoadjuvant chemotherapy: an overview with recommendations on specimen processing and reporting. Arch Pathol Lab Med. 2009; 133:633–42.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Neoadjuvant Therapy Should Be the Standard of Care for Every Node Positive Breast Cancer Patient
- Systemic adjuvant therapy in breast cancer
- Comment on “Histomorphological Factors Predicting the Response to Neoadjuvant Chemotherapy in Triple-Negative Breast Cancerâ€
- Evaluation of Prognostic Factors and Validation of Tumor Response Ratios after Neoadjuvant Chemotherapy in Patients with Breast Cancer
- Recent Advances in the Neoadjuvant Treatment of Breast Cancer