Korean J Urol.
2015 Aug;56(8):587-593. 10.4111/kju.2015.56.8.587.
Evaluation of low-dose dual energy computed tomography for in vivo assessment of renal/ureteric calculus composition
- Affiliations
-
- 1Department of Radiodiagnosis, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. dralal@rediffmail.com
- 2Department of Urology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India.
- 3Department of Biophysics, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India.
- KMID: 2164410
- DOI: http://doi.org/10.4111/kju.2015.56.8.587
Abstract
- PURPOSE
This study aimed to assess the accuracy of low-dose dual-energy computed tomography (DECT) in predicting the composition of urinary calculi.
MATERIALS AND METHODS
A total of 52 patients with urinary calculi were scanned with a 128-slice dual-source DECT scanner by use of a low-dose protocol. Dual-energy (DE) ratio, weighted average Hounsfield unit (HU) of calculi, radiation dose, and image noise levels were recorded. Two radiologists independently rated study quality. Stone composition was assessed after extraction by Fourier transform infrared spectroscopy (FTIRS). Analysis of variance was used to determine if the differences in HU values and DE ratios between the various calculus groups were significant. Threshold cutoff values to classify the calculi into separate groups were identified by receiver operating characteristic curve analysis.
RESULTS
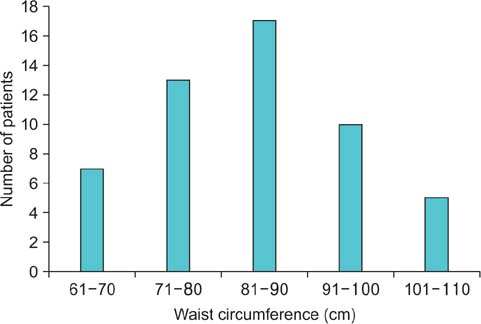
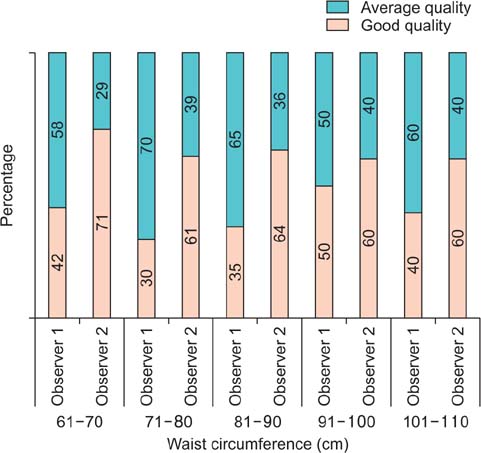
A total of 137 calculi were detected. FTIRS analysis differentiated the calculi into five groups: uric acid (n=17), struvite (n=3), calcium oxalate monohydrate and dihydrate (COM-COD, n=84), calcium oxalate monohydrate (COM, n=28), and carbonate apatite (n=5). The HU value could differentiate only uric acid calculi from calcified calculi (p<0.001). The DE ratio could confidently differentiate uric acid, struvite, calcium oxalate, and carbonate apatite calculi (p<0.001) with cutoff values of 1.12, 1.34, and 1.66, respectively, giving >80% sensitivity and specificity to differentiate them. The DE ratio could not differentiate COM from COM-COD calculi. No study was rated poor in quality by either of the observers. The mean radiation dose was 1.8 mSv.
CONCLUSIONS
Low-dose DECT accurately predicts urinary calculus composition in vivo while simultaneously reducing radiation exposure without compromising study quality.
MeSH Terms
-
Adult
Apatites/analysis
Calcium Oxalate/analysis
Female
Humans
Image Interpretation, Computer-Assisted/methods
Kidney Calculi/chemistry/pathology/*radiography
Magnesium Compounds/analysis
Male
Middle Aged
Phosphates/analysis
Prospective Studies
Radiation Dosage
Tomography, X-Ray Computed/methods
Ureteral Calculi/chemistry/pathology/*radiography
Uric Acid/analysis
Waist Circumference
Young Adult
Apatites
Calcium Oxalate
Magnesium Compounds
Phosphates
Uric Acid
Figure
Reference
-
1. Turk C, Knoll T, Petrik A, Sarica K, Straub M, Seitz C. Guidelines on urolithiasis [Internet]. ARNHEM (NL): European Association of Urology;c2015. cited 2015 Mar 7. Available from: uroweb.org/guideline/urolithiasis/.2. Torricelli FC, De S, Liu X, Calle J, Gebreselassie S, Monga M. Can 24-hour urine stone risk profiles predict urinary stone composition? J Endourol. 2014; 28:735–738.3. Joseph P, Mandal AK, Singh SK, Mandal P, Sankhwar SN, Sharma SK. Computerized tomography attenuation value of renal calculus: can it predict successful fragmentation of the calculus by extracorporeal shock wave lithotripsy? A preliminary study. J Urol. 2002; 167:1968–1971.4. Anastasiadis A, Onal B, Modi P, Turna B, Duvdevani M, Timoney A, et al. Impact of stone density on outcomes in percutaneous nephrolithotomy (PCNL): an analysis of the clinical research office of the endourological society (CROES) pcnl global study database. Scand J Urol. 2013; 47:509–514.5. Primak AN, Fletcher JG, Vrtiska TJ, Dzyubak OP, Lieske JC, Jackson ME, et al. Noninvasive differentiation of uric acid versus non-uric acid kidney stones using dual-energy CT. Acad Radiol. 2007; 14:1441–1447.6. Matlaga BR, Kawamoto S, Fishman E. Dual source computed tomography: a novel technique to determine stone composition. Urology. 2008; 72:1164–1168.7. Manglaviti G, Tresoldi S, Guerrer CS, Di Leo G, Montanari E, Sardanelli F, et al. In vivo evaluation of the chemical composition of urinary stones using dual-energy CT. AJR Am J Roentgenol. 2011; 197:W76–W83.8. Qu M, Ramirez-Giraldo JC, Leng S, Williams JC, Vrtiska TJ, Lieske JC, et al. Dual-energy dual-source CT with additional spectral filtration can improve the differentiation of non-uric acid renal stones: an ex vivo phantom study. AJR Am J Roentgenol. 2011; 196:1279–1287.9. Fung GS, Kawamoto S, Matlaga BR, Taguchi K, Zhou X, Fishman EK, et al. Differentiation of kidney stones using dualenergy CT with and without a tin filter. AJR Am J Roentgenol. 2012; 198:1380–1386.10. Acharya S, Goyal A, Bhalla AS, Sharma R, Seth A, Gupta AK. In vivo characterization of urinary calculi on dual-energy CT: going a step ahead with sub-differentiation of calcium stones. Acta Radiol. 2015; 56:881–889.11. Thomas C, Krauss B, Ketelsen D, Tsiflikas I, Reimann A, Werner M, et al. Differentiation of urinary calculi with dual energy CT: effect of spectral shaping by high energy tin filtration. Invest Radiol. 2010; 45:393–398.12. Huda W, Ogden KM, Khorasani MR. Converting dose-length product to effective dose at CT. Radiology. 2008; 248:995–1003.13. Cohen TD, Preminger GM. Struvite calculi. Semin Nephrol. 1996; 16:425–434.14. Zanetti G, Paparella S, Trinchieri A, Prezioso D, Rocco F, Naber KG. Infections and urolithiasis: current clinical evidence in prophylaxis and antibiotic therapy. Arch Ital Urol Androl. 2008; 80:5–12.15. Evan AP, Lingeman J, Coe F, Shao Y, Miller N, Matlaga B, et al. Renal histopathology of stone-forming patients with distal renal tubular acidosis. Kidney Int. 2007; 71:795–801.16. Mettler FA Jr, Huda W, Yoshizumi TT, Mahesh M. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008; 248:254–263.17. Pierce DA, Preston DL. Radiation-related cancer risks at low doses among atomic bomb survivors. Radiat Res. 2000; 154:178–186.18. Thomas C, Heuschmid M, Schilling D, Ketelsen D, Tsiflikas I, Stenzl A, et al. Urinary calculi composed of uric acid, cystine, and mineral salts: differentiation with dual-energy CT at a radiation dose comparable to that of intravenous pyelography. Radiology. 2010; 257:402–409.19. Thomas C, Patschan O, Ketelsen D, Tsiflikas I, Reimann A, Brodoefel H, et al. Dual-energy CT for the characterization of urinary calculi: In vitro and in vivo evaluation of a low-dose scanning protocol. Eur Radiol. 2009; 19:1553–1559.20. Ascenti G, Siragusa C, Racchiusa S, Ielo I, Privitera G, Midili F, et al. Stone-targeted dual-energy CT: a new diagnostic approach to urinary calculosis. AJR Am J Roentgenol. 2010; 195:953–958.21. Thomson JM, Glocer J, Abbott C, Maling TM, Mark S. Computed tomography versus intravenous urography in diagnosis of acute flank pain from urolithiasis: a randomized study comparing imaging costs and radiation dose. Australas Radiol. 2001; 45:291–297.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of Radiation Dose for Dual Energy CBCT Using Multi-Grid Device
- Various Methods of Analysing Body Composition
- Use of Dual-Energy X-ray Absorptiometry for Body Composition in Chronic Disease Management
- Imaging Techniques for Nutritional Assessment
- Effectiveness of Virtual Non-Enhanced Images Acquired by Dual-Energy Computed Tomography in Evaluation of Patients with Suspected Acute Appendicitis