The Korean guideline for cervical cancer screening
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University Medical Center, Seoul, Korea. jklee38@gmail.com
- 2Department of Korean Gynecology, Jaseng Hospital of Korean Medicine, Seoul, Korea.
- 3National Cancer Control Institute, National Cancer Center, Goyang, Korea.
- 4Center for Uterine Cancer and Department of Pathology, National Cancer Center, Goyang, Korea.
- 5Center for Uterine Cancer, Hospital, Gynecologic Cancer Branch, Research Institute, Department of Cancer Control and Policy, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea.
- 6Department of Family Medicine, Konkuk University Medical Center, Konkuk University School of Medicine, Seoul, Korea.
- 7Department of Cancer Control and Policy, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea.
- 8Department of Obstetrics and Gynecology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 9Department of Obstetrics and Gynecology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea.
- 10Department of Obstetrics and Gynecology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 11Department of Family Medicine, Dankook University College of Medicine, Cheonan, Korea.
- 12Department of Social Medicine, Dankook University College of Medicine, Cheonan, Korea.
- 13Department of Pathology, Chosun University School of Medicine, Gwangju, Korea.
- 14Department of Obstetrics and Gynecology, Keimyung University School of Medicine, Daegu, Korea.
- 15Department of Pathology, Cheil General Hospital & Women's Healthcare Center, Dankook University College of Medicine, Seoul, Korea.
- 16Department of Family Medicine, Hallym University Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- 17Department of Preventive Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- KMID: 2160811
- DOI: http://doi.org/10.3802/jgo.2015.26.3.232
Abstract
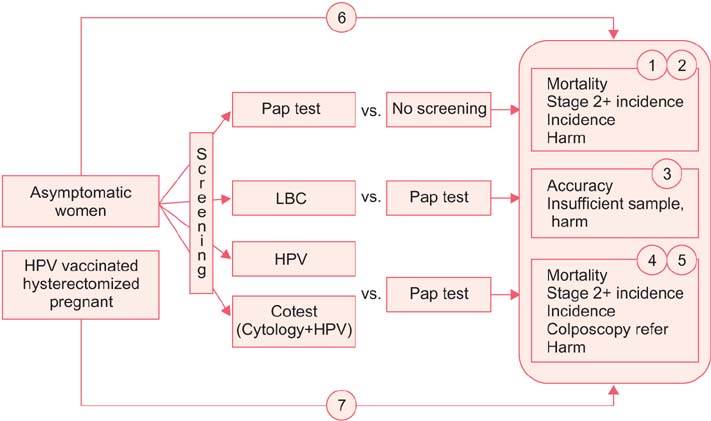
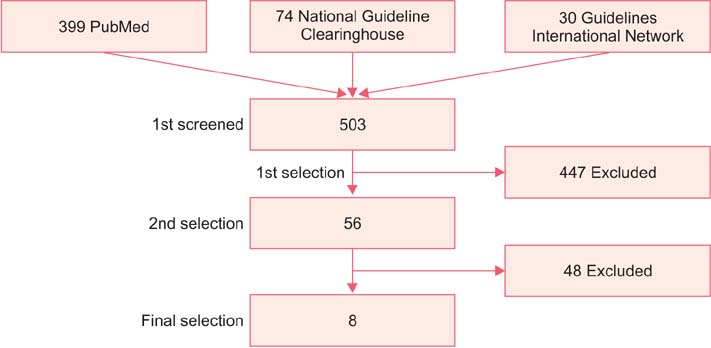
- The incidence rate of cervical cancer in Korea is still higher than in other developed countries, notwithstanding the national mass-screening program. Furthermore, a new method has been introduced in cervical cancer screening. Therefore, the committee for cervical cancer screening in Korea updated the recommendation statement established in 2002. The new version of the guideline was developed by the committee using evidence-based methods. The committee reviewed the evidence for the benefits and harms of the Papanicolaou test, liquid-based cytology, and human papillomavirus (HPV) testing, and reached conclusions after deliberation. The committee recommends screening for cervical cancer with cytology (Papanicolaou test or liquid-based cytology) every three years in women older than 20 years of age (recommendation A). The cervical cytology combined with HPV test is optionally recommended after taking into consideration individual risk or preference (recommendation C). The current evidence for primary HPV screening is insufficient to assess the benefits and harms of cervical cancer screening (recommendation I). Cervical cancer screening can be terminated at the age of 74 years if more than three consecutive negative cytology reports have been confirmed within 10 years (recommendation D).
MeSH Terms
-
Adult
Age Factors
Aged
Early Detection of Cancer/adverse effects/*methods/standards
Evidence-Based Medicine
False Positive Reactions
Female
Humans
Hysterectomy
Middle Aged
Papillomavirus Infections/diagnosis
Papillomavirus Vaccines
Patient Selection
Pregnancy
Pregnancy Complications, Neoplastic/diagnosis
Republic of Korea
Review Literature as Topic
Uterine Cervical Neoplasms/*diagnosis
Vaginal Smears/adverse effects/methods/standards
Young Adult
Papillomavirus Vaccines
Figure
Cited by 11 articles
-
Proposal for cervical cancer screening in the era of HPV vaccination
Yung-Taek Ouh, Jae Kwan Lee
Obstet Gynecol Sci. 2018;61(3):298-308. doi: 10.5468/ogs.2018.61.3.298.Prevalence of human papillomavirus genotypes and precancerous cervical lesions in a screening population in the Republic of Korea, 2014–2016
Yung-Taek Ouh, Kyung-Jin Min, Hyun Woong Cho, Moran Ki, Jin-Kyoung Oh, Sang Yop Shin, Jin Hwa Hong, Jae-Kwan Lee
J Gynecol Oncol. 2018;29(1):. doi: 10.3802/jgo.2018.29.e14.Impact of adjuvant hysterectomy on prognosis in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy: a meta-analysis
Seung-Hyuk Shim, Soo-Nyung Kim, Su Hyun Chae, Jung Eun Kim, Sun Joo Lee
J Gynecol Oncol. 2018;29(2):. doi: 10.3802/jgo.2018.29.e25.Clinical and health policy experiences with HPV prevalence within the Hungarian organized cervical cancer screening program
Réka Vajda, Krisztina Juhász, Dóra Endrei
J Gynecol Oncol. 2018;29(3):. doi: 10.3802/jgo.2018.29.e45.High-risk human papillomavirus testing as a primary screening for cervical cancer: position statement by the Korean Society of Obstetrics and Gynecology and the Korean Society of Gynecologic Oncology
Tae-Wook Kong, Miseon Kim, Young-Han Kim, Yong Beom Kim, Jayeon Kim, Jae-Weon Kim, Mi Hye Park, Joo Hyun Park, Jeong Ho Rhee, Myong Cheol Lim, Joon-Seok Hong
J Gynecol Oncol. 2020;31(1):. doi: 10.3802/jgo.2020.31.e31.Discrepancy between Cytology and Histology in Cervical Cancer Screening: a Multicenter Retrospective Study (KGOG 1040)
Yung-Taek Ouh, Ji Jeong Park, Minjoo Kang, Miseon Kim, Jae Yun Song, So Jin Shin, Seung-Hyuk Shim, Heon Jong Yoo, Maria Lee, Sung-Jong Lee, Whan Shin, Gun Oh Chong, Min Chul Choi, Chel Hun Choi, Kyung-Jin Min
J Korean Med Sci. 2021;36(24):e164. doi: 10.3346/jkms.2021.36.e164.Comparison of Unsatisfactory Samples from Conventional Smear versus Liquid-Based Cytology in Uterine Cervical Cancer Screening Test
Hoiseon Jeong, Sung Ran Hong, Seoung-Wan Chae, So-Young Jin, Hye Kyoung Yoon, Juhie Lee, Eun Kyung Kim, Sook Tai Ha, Sung Nam Kim, Eun-Jung Park, Jong Jae Jung, Sun Hee Sung, Sung-chul Lim
J Pathol Transl Med. 2017;51(3):314-319. doi: 10.4132/jptm.2017.03.17.Current Cytology Practices in Korea: A Nationwide Survey by the Korean Society for Cytopathology
Eun Ji Oh, Chan Kwon Jung, Dong-Hoon Kim, Han Kyeom Kim, Wan Seop Kim, So-Young Jin, Hye Kyoung Yoon
J Pathol Transl Med. 2017;51(6):579-587. doi: 10.4132/jptm.2017.08.11.Current Status of and Perspectives on Cervical Cancer Screening in Korea
Sung-Chul Lim, Chong Woo Yoo
J Pathol Transl Med. 2019;53(4):210-216. doi: 10.4132/jptm.2019.04.11.Clinical management of abnormal Pap tests: differences between US and Korean guidelines
Seyeon Won, Mi Kyoung Kim, Seok Ju Seong
J Pathol Transl Med. 2020;54(3):213-219. doi: 10.4132/jptm.2020.03.11.Cervical Cancer in Women with Normal Papanicolaou Tests: A Korean Nationwide Cohort Study
Miseon Kim, Hyeongsu Kim, Dong Hoon Suh, Yong Beom Kim
Cancer Res Treat. 2021;53(3):813-818. doi: 10.4143/crt.2020.826.
Reference
-
1. National Cancer Control Institute. National Cancer Incidence 2012, Korea. Goyang: Ministry of Health and Welfare;2014.2. Moyer VA. U.S. Preventive Services Task Force. Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012; 156:880–891. W3123. Canadian Task Force on Preventive Health Care. Dickinson J, Tsakonas E, Conner Gorber S, Lewin G, Shaw E, et al. Recommendations on screening for cervical cancer. CMAJ. 2013; 185:35–45.4. Lee JK, Hong JH, Kang S, Kim DY, Kim BG, Kim SH, et al. Practice guidelines for the early detection of cervical cancer in Korea: Korean Society of Gynecologic Oncology and the Korean Society for Cytopathology 2012 edition. J Gynecol Oncol. 2013; 24:186–203.5. Saslow D, Solomon D, Lawson HW, Killackey M, Kulasingam SL, Cain J, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012; 62:147–172.6. Committee on Practice Bulletins-Gynecology. ACOG Practice Bulletin Number 131: screening for cervical cancer. Obstet Gynecol. 2012; 120:1222–1238.7. Murphy J, Kennedy EB, Dunn S, McLachlin CM, Fung Kee Fung M, Gzik D, et al. Cervical screening: a guideline for clinical practice in Ontario. J Obstet Gynaecol Can. 2012; 34:453–458.8. Partridge EE, Abu-Rustum NR, Campos SM, Fahey PJ, Farmer M, Garcia RL, et al. Cervical cancer screening. J Natl Compr Canc Netw. 2010; 8:1358–1386.9. Hamashima C, Aoki D, Miyagi E, Saito E, Nakayama T, Sagawa M, et al. The Japanese guideline for cervical cancer screening. Jpn J Clin Oncol. 2010; 40:485–502.10. Royal Australian College of General Practitioners. Early detection of cancers. Guidelines for preventive activities in general practice. 8th ed. East Melbourne: Royal Australian College of General Practitioners;2012. p. 60–72.11. Brouwers MC, Kho ME, Browman GP, Burgers JS, Cluzeau F, Feder G, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010; 182:E839–E842.12. Kim SY, Park JE, Lee YJ, Seo HJ, Sheen SS, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013; 66:408–414.13. Shea BJ, Hamel C, Wells GA, Bouter LM, Kristjansson E, Grimshaw J, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009; 62:1013–1020.14. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011; 64:383–394.15. Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med. 2009; 360:1385–1394.16. Herbert A, Stein K, Bryant TN, Breen C, Old P. Relation between the incidence of invasive cervical cancer and the screening interval: is a five year interval too long? J Med Screen. 1996; 3:140–145.17. Andrae B, Kemetli L, Sparen P, Silfverdal L, Strander B, Ryd W, et al. Screening-preventable cervical cancer risks: evidence from a nationwide audit in Sweden. J Natl Cancer Inst. 2008; 100:622–629.18. Aristizabal N, Cuello C, Correa P, Collazos T, Haenszel W. The impact of vaginal cytology on cervical cancer risks in Cali, Colombia. Int J Cancer. 1984; 34:5–9.19. Berrino F, Gatta G, d'Alto M, Crosignani P, Riboli E. Efficacy of screening in preventing invasive cervical cancer: a case-control study in Milan, Italy. IARC Sci Publ. 1986; (76):111–123.20. Clarke EA, Anderson TW. Does screening by "Pap" smears help prevent cervical cancer? A case-control study. Lancet. 1979; 2:1–4.21. Decker K, Demers A, Chateau D, Musto G, Nugent Z, Lotocki R, et al. Papanicolaou test utilization and frequency of screening opportunities among women diagnosed with cervical cancer. Open Med. 2009; 3:e140–e147.22. Hernandez-Avila M, Lazcano-Ponce EC, de Ruiz PA, Romieu I. Evaluation of the cervical cancer screening programme in Mexico: a population-based case-control study. Int J Epidemiol. 1998; 27:370–376.23. Herrero R, Brinton LA, Reeves WC, Brenes MM, de Britton RC, Gaitan E, et al. Screening for cervical cancer in Latin America: a case-control study. Int J Epidemiol. 1992; 21:1050–1056.24. Hoffman M, Cooper D, Carrara H, Rosenberg L, Kelly J, Stander I, et al. Limited Pap screening associated with reduced risk of cervical cancer in South Africa. Int J Epidemiol. 2003; 32:573–577.25. Jimenez-Perez M, Thomas DB. Has the use of pap smears reduced the risk of invasive cervical cancer in Guadalajara, Mexico? Int J Cancer. 1999; 82:804–809.26. Makino H, Sato S, Yajima A, Komatsu S, Fukao A. Evaluation of the effectiveness of cervical cancer screening: a case-control study in Miyagi, Japan. Tohoku J Exp Med. 1995; 175:171–178.27. Nieminen P, Kallio M, Anttila A, Hakama M. Organised vs. spontaneous Pap-smear screening for cervical cancer: a case-control study. Int J Cancer. 1999; 83:55–58.28. Talbott EO, Norman SA, Kuller LH, Ishii EK, Baffone KM, Dunn MS, et al. Refining preventive strategies for invasive cervical cancer: a population-based case-control study. J Womens Health. 1995; 4:387–395.29. Cho BR. Evaluation of the validity of current national health screening program and plan to improve the system. Cheonju: Korea Centers for Disease Control and Prevention;2013.30. Jun JK, Choi KS, Jung KW, Lee HY, Gapstur SM, Park EC, et al. Effectiveness of an organized cervical cancer screening program in Korea: results from a cohort study. Int J Cancer. 2009; 124:188–193.31. Siebers AG, Klinkhamer PJ, Grefte JM, Massuger LF, Vedder JE, Beijers-Broos A, et al. Comparison of liquid-based cytology with conventional cytology for detection of cervical cancer precursors: a randomized controlled trial. JAMA. 2009; 302:1757–1764.32. Ronco G, Cuzick J, Pierotti P, Cariaggi MP, Dalla Palma P, Naldoni C, et al. Accuracy of liquid based versus conventional cytology: overall results of new technologies for cervical cancer screening: randomised controlled trial. BMJ. 2007; 335:28.33. Taylor S, Kuhn L, Dupree W, Denny L, De Souza M, Wright TC Jr. Direct comparison of liquid-based and conventional cytology in a South African screening trial. Int J Cancer. 2006; 118:957–962.34. Coste J, Cochand-Priollet B, de Cremoux P, Le Gales C, Cartier I, Molinie V, et al. Cross sectional study of conventional cervical smear, monolayer cytology, and human papillomavirus DNA testing for cervical cancer screening. BMJ. 2003; 326:733.35. Ko MJ, Kim Y, Hong SR, Lee JK, Shim J, Kim J, et al. Cost-effectiveness of conventional cytology and HPV DNA testing for cervical cancer screening in South Korea. Seoul: National Evidence-based Healthcare Collaborating Agency;2014.36. Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010; 11:249–257.37. Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007; 370:1764–1772.38. Naucler P, Ryd W, Tornberg S, Strand A, Wadell G, Elfgren K, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007; 357:1589–1597.39. Kitchener HC, Almonte M, Thomson C, Wheeler P, Sargent A, Stoykova B, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol. 2009; 10:672–682.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New insights into cervical cancer screening
- Revision process of and expert committee composition for Korean national cancer screening guideline
- Guidelines for the Screening of Uterine Cervical Cancer
- Epidemiologic characteristics of cervical cancer in Korean women
- Related Factors to Screening or Repeat Screening for Cervical and Breast Cancer among Women