J Korean Med Assoc.
2015 Apr;58(4):277-283. 10.5124/jkma.2015.58.4.277.
Revision process of and expert committee composition for Korean national cancer screening guideline
- Affiliations
-
- 1National Cancer Control Institute, Goyang, Korea. leedukh@ncc.re.kr
- 2Center for Cancer Detection & Prevention, National Cancer Center, Goyang, Korea.
- 3Department of Preventive Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 4Department of Family Medicine, Hallym University Kangdong Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea.
- KMID: 2194959
- DOI: http://doi.org/10.5124/jkma.2015.58.4.277
Abstract
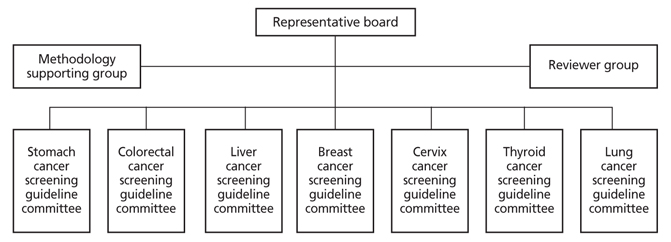
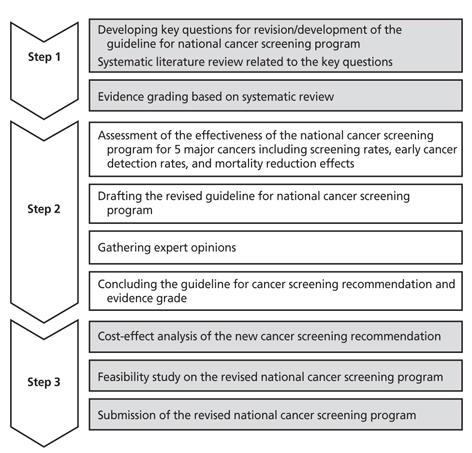
- Cancer screening is one of the most effective methods for cancer control. The national cancer screening program has provided regular cancer screenings for all people at a certain age, regardless of symptoms. This program covers five major cancers: stomach, colorectal, liver, breast, and cervical cancer. Recently, a research project was performed to develop and revise the guidelines for cancer screening, based on the assessment of effectiveness compared to harm and on the evidence from a systematic review of related studies. Target cancers for screening guideline are not only for five major cancers which are included in national cancer screening program, but also for thyroid cancer and lung cancer, because thyroid cancer is rapidly increased recently and lung cancer has the highest mortality rate among cancers. Multidisciplinary expert committees were composed for developing and revising the guidelines for cancer screening. This process of national cancer screening guideline development and revision comprised three steps. First, an expert committee developed key questions for consideration in revision and development of the guidelines. A systematic literature review related to these key questions was performed. In the second step, the effectiveness of the national cancer screening program for five major cancers was analyzed, including analysis of screening rates, early cancer detection rates, and mortality reduction effects. Through this process, a draft of the revised guidelines was created. The draft was open to the public to gather external expert opinions. After review of the expert opinions, the final guidelines for cancer screening were published. In the third step, based on the revised cancer screening guideline, the national cancer screening program will be modified. In this step, cost-effectiveness and feasibility of the revised guideline will be considered.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Background and significance of Korean national cancer screening guideline revision
Won-Chul Lee, Yeol Kim
J Korean Med Assoc. 2015;58(4):274-276. doi: 10.5124/jkma.2015.58.4.274.
Reference
-
1. Barratt A, Irwig L, Glasziou P, Cumming RG, Raffle A, Hicks N, Gray JA, Guyatt GH. Evidence-Based Medicine Working Group. Users' guides to the medical literature: XVII. How to use guidelines and recommendations about screening. JAMA. 1999; 281:2029–2034.2. Raffle AE, Gray JA. Screening: evidence and practice. Oxford: Oxford University Press;2007.3. Hamashima C, Saito H, Nakayama T, Nakayama T, Sobue T. The standardized development method of the Japanese guidelines for cancer screening. Jpn J Clin Oncol. 2008; 38:288–295.
Article4. Zaza S, Lawrence RS, Mahan CS, Fullilove M, Fleming D, Isham GJ, Pappaioanou M. Scope and organization of the Guide to Community Preventive Services: the Task Force on Commu-nity Preventive Services. Am J Prev Med. 2000; 18:1 Suppl. 27–34.
Article5. Franco EL, Duarte-Franco E, Rohan TE. Evidence-based policy recommendations on cancer screening and prevention. Cancer Detect Prev. 2002; 26:350–361.
Article6. UK National Screening Committee. UK screening portal [Internet]. London: UK National Screening Committee;2015. cited 2015 Feb 20. Available from: http://www.screening.nhs.uk/uknsc.7. Canadian Task Force on Preventive Health Care. Appraised guidelines [Internet]. Calgary: Canadian Task Force on Preven-tive Health Care;2015. cited 2015 Feb 20. Available from: http://canadiantaskforce.ca/appraised-guidelines/overview/.8. Neuman J, Korenstein D, Ross JS, Keyhani S. Prevalence of financial conflicts of interest among panel members producing clinical practice guidelines in Canada and United States: cross sectional study. BMJ. 2011; 343:d5621.
Article9. Petitti DB, Teutsch SM, Barton MB, Sawaya GF, Ockene JK, DeWitt T. U.S. Preventive Services Task Force. Update on the methods of the U.S. Preventive Services Task Force: insufficient evidence. Ann Intern Med. 2009; 150:199–205.
Article10. Vandvik PO, Santesso N, Akl EA, You J, Mulla S, Spencer FA, Johnston BC, Brozek J, Kreis J, Brandt L, Zhou Q, Schunemann HJ, Guyatt G. Formatting modifications in GRADE evidence profiles improved guideline panelists comprehension and acce-ssibility to information: a randomized trial. J Clin Epidemiol. 2012; 65:748–755.
Article11. Cancer Australia. Australian population health development principal committee: screening subcommittee [Internet]. Surry Hills: Cancer Australia;2015. cited 2015 Feb 20. Available from: http://canceraustralia.gov.au.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 2018 Korean Liver Cancer Association–National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma
- 2018 Korean Liver Cancer Association–National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma
- Revision of Quality Indicators for the Endoscopy Quality Improvement Program of the National Cancer Screening Program in Korea
- Revision of Quality Indicators for the Endoscopy Quality Improvement Program of the National Cancer Screening Program in Korea
- 2022 KLCA-NCC Korea practice guidelines for the management of hepatocellular carcinoma