J Gynecol Oncol.
2015 Jan;26(1):19-24. 10.3802/jgo.2015.26.1.19.
The incidence of pelvic and para-aortic lymph node metastasis in uterine papillary serous and clear cell carcinoma according to the SEER registry
- Affiliations
-
- 1Department of Radiation Oncology, West Virginia University, Mograntown, WV, USA. malcolm.mattes@gmail.com
- 2Department of Radiation Oncology, New York Methodist Hospital, Brooklyn, NY, USA.
- KMID: 2158798
- DOI: http://doi.org/10.3802/jgo.2015.26.1.19
Abstract
OBJECTIVE
In this study we utilized the Surveillance, Epidemiology and End-Results (SEER) registry to identify risk factors for lymphatic spread and determine the incidence of pelvic and para-aortic lymph node metastases in patients with uterine papillary serous carcinoma (UPSC) and uterine clear cell carcinoma (UCCC) who underwent complete surgical staging and lymph node dissection.
METHODS
Nine hundred seventy-two eligible patients diagnosed between 1998 to 2009 with International Federation of Gynecology and Obstetrics (FIGO) 1988 stage IA-IVA UPSC (n=685) or UCCC (n=287) were identified for analysis. Binomial logistic regression was used to determine risk factors for lymph node metastasis, with the incidence of pelvic and para-aortic lymph node metastases reported for each FIGO primary tumor stage. The Cox proportional hazards regression model was used to determine factors associated with overall survival.
RESULTS
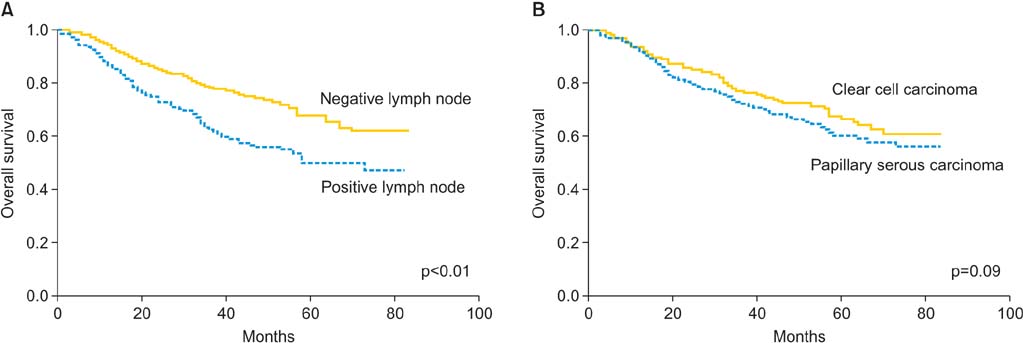
FIGO primary tumor stage was the only independent risk factor for lymph node metastasis (p<0.01). The incidence of pelvis-only and para-aortic lymph node involvement according to the FIGO primary tumor stage were as follows: IA (2.3%/3.8%), IB (7.5%/5.2%), IC (22.5%/16.9%), IIA (20.8%/13.2%), IIB (25.7%/14.9%), and III/IV (25.7%/24.3%). Prognostic factors for overall survival included lymph node involvement (hazard ratio [HR], 1.42; 95% confidence interval [CI], 1.09 to 1.85; p<0.01), patient age >60 years (HR, 1.70; 95% CI, 1.21 to 2.41; p<0.01), and advanced FIGO primary tumor stage (p<0.01). Tumor grade, histologic subtype, and patient race did not predict for either lymph node metastasis or overall survival.
CONCLUSION
There is a high incidence of both pelvic and para-aortic lymph node metastases for FIGO stages IC and above uterine papillary serous and clear cell carcinomas, suggesting a potential role for lymph node-directed therapy for these patients.
MeSH Terms
-
Adenocarcinoma, Clear Cell/epidemiology/pathology/*secondary/surgery
Adult
Aged
Aged, 80 and over
Aorta, Abdominal
Cystadenocarcinoma, Papillary/epidemiology/pathology/*secondary/surgery
Cystadenocarcinoma, Serous/epidemiology/pathology/*secondary/surgery
Female
Humans
Incidence
Kaplan-Meier Estimate
Lymph Node Excision
Lymphatic Metastasis
Middle Aged
Neoplasm Grading
Neoplasm Staging
Pelvis
SEER Program
United States/epidemiology
Uterine Neoplasms/*epidemiology/pathology/surgery
Figure
Reference
-
1. del Carmen MG, Birrer M, Schorge JO. Uterine papillary serous cancer: a review of the literature. Gynecol Oncol. 2012; 127:651–661.2. Fader AN, Boruta D, Olawaiye AB, Gehrig PA. Uterine papillary serous carcinoma: epidemiology, pathogenesis and management. Curr Opin Obstet Gynecol. 2010; 22:21–29.3. Gadducci A, Cosio S, Spirito N, Cionini L. Clear cell carcinoma of the endometrium: a biological and clinical enigma. Anticancer Res. 2010; 30:1327–1334.4. Mendivil A, Schuler KM, Gehrig PA. Non-endometrioid adenocarcinoma of the uterine corpus: a review of selected histological subtypes. Cancer Control. 2009; 16:46–52.5. Carcangiu ML, Tan LK, Chambers JT. Stage IA uterine serous carcinoma: a study of 13 cases. Am J Surg Pathol. 1997; 21:1507–1514.6. Gehrig PA, Groben PA, Fowler WC Jr, Walton LA, Van Le L. Noninvasive papillary serous carcinoma of the endometrium. Obstet Gynecol. 2001; 97:153–157.7. Goff BA, Kato D, Schmidt RA, Ek M, Ferry JA, Muntz HG, et al. Uterine papillary serous carcinoma: patterns of metastatic spread. Gynecol Oncol. 1994; 54:264–268.8. Hui P, Kelly M, O'Malley DM, Tavassoli F, Schwartz PE. Minimal uterine serous carcinoma: a clinicopathological study of 40 cases. Mod Pathol. 2005; 18:75–82.9. Fader AN, Starks D, Gehrig PA, Secord AA, Frasure HE, O'Malley DM, et al. An updated clinicopathologic study of early-stage uterine papillary serous carcinoma (UPSC). Gynecol Oncol. 2009; 115:244–248.10. Halperin R, Zehavi S, Langer R, Hadas E, Bukovsky I, Schneider D. Uterine papillary serous carcinoma (pure and mixed type) compared with moderately and poorly differentiated endometrioid carcinoma: a clinicopathologic study. Eur J Gynaecol Oncol. 2002; 23:300–304.11. Silva EG, Jenkins R. Serous carcinoma in endometrial polyps. Mod Pathol. 1990; 3:120–128.12. Wheeler DT, Bell KA, Kurman RJ, Sherman ME. Minimal uterine serous carcinoma: diagnosis and clinicopathologic correlation. Am J Surg Pathol. 2000; 24:797–806.13. Chang-Halpenny CN, Natarajan S, Hwang-Graziano J. Early stage papillary serous or clear cell carcinoma confined to or involving an endometrial polyp: outcomes with and without adjuvant therapy. Gynecol Oncol. 2013; 131:598–603.14. Slomovitz BM, Burke TW, Eifel PJ, Ramondetta LM, Silva EG, Jhingran A, et al. Uterine papillary serous carcinoma (UPSC): a single institution review of 129 cases. Gynecol Oncol. 2003; 91:463–469.15. Lee KR, Belinson JL. Recurrence in noninvasive endometrial carcinoma: relationship to uterine papillary serous carcinoma. Am J Surg Pathol. 1991; 15:965–973.16. Alektiar KM, McKee A, Lin O, Venkatraman E, Zelefsky MJ, McKee B, et al. Is there a difference in outcome between stage I-II endometrial cancer of papillary serous/clear cell and endometrioid FIGO Grade 3 cancer? Int J Radiat Oncol Biol Phys. 2002; 54:79–85.17. Cirisano FD Jr, Robboy SJ, Dodge RK, Bentley RC, Krigman HR, Synan IS, et al. Epidemiologic and surgicopathologic findings of papillary serous and clear cell endometrial cancers when compared to endometrioid carcinoma. Gynecol Oncol. 1999; 74:385–394.18. Greggi S, Mangili G, Scaffa C, Scala F, Losito S, Iodice F, et al. Uterine papillary serous, clear cell, and poorly differentiated endometrioid carcinomas: a comparative study. Int J Gynecol Cancer. 2011; 21:661–667.19. Abu-Rustum NR, Iasonos A, Zhou Q, Oke E, Soslow RA, Alektiar KM, et al. Is there a therapeutic impact to regional lymphadenectomy in the surgical treatment of endometrial carcinoma? Am J Obstet Gynecol. 2008; 198:457.e1. 457.e5.20. Cragun JM, Havrilesky LJ, Calingaert B, Synan I, Secord AA, Soper JT, et al. Retrospective analysis of selective lymphadenectomy in apparent early-stage endometrial cancer. J Clin Oncol. 2005; 23:3668–3675.21. Mikuta JJ. International Federation of Gynecology and Obstetrics staging of endometrial cancer 1988. Cancer. 1993; 71:4 Suppl. 1460–1463.22. Sutton G, Axelrod JH, Bundy BN, Roy T, Homesley H, Lee RB, et al. Adjuvant whole abdominal irradiation in clinical stages I and II papillary serous or clear cell carcinoma of the endometrium: a phase II study of the Gynecologic Oncology Group. Gynecol Oncol. 2006; 100:349–354.23. Kwon J, Ackerman I, Franssen E. The role of abdominal-pelvic radiotherapy in the management of uterine papillary serous carcinoma. Int J Radiat Oncol Biol Phys. 2004; 59:1439–1445.24. Lim P, Al Kushi A, Gilks B, Wong F, Aquino-Parsons C. Early stage uterine papillary serous carcinoma of the endometrium: effect of adjuvant whole abdominal radiotherapy and pathologic parameters on outcome. Cancer. 2001; 91:752–757.25. Martinez AA, Weiner S, Podratz K, Armin AR, Stromberg JS, Stanhope R, et al. Improved outcome at 10 years for serouspapillary/clear cell or high-risk endometrial cancer patients treated by adjuvant high-dose whole abdomino-pelvic irradiation. Gynecol Oncol. 2003; 90:537–546.26. Mehta N, Yamada SD, Rotmensch J, Mundt AJ. Outcome and pattern of failure in pathologic stage I-II papillary serous carcinoma of the endometrium: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2003; 57:1004–1009.27. Greven K, Winter K, Underhill K, Fontenesci J, Cooper J, Burke T. Final analysis of RTOG 9708: adjuvant postoperative irradiation combined with cisplatin/paclitaxel chemotherapy following surgery for patients with high-risk endometrial cancer. Gynecol Oncol. 2006; 103:155–159.28. Fishman A, Altaras M, Bernheim J, Cohen I, Beyth Y, Tepper R. The value of transvaginal sonography in the preoperative assessment of myometrial invasion in high and low grade endometrial cancer and in comparison to frozen section in grade 1 disease. Eur J Gynaecol Oncol. 2000; 21:128–130.29. Case AS, Rocconi RP, Straughn JM Jr, Conner M, Novak L, Wang W, et al. A prospective blinded evaluation of the accuracy of frozen section for the surgical management of endometrial cancer. Obstet Gynecol. 2006; 108:1375–1379.30. Gilks CB, Oliva E, Soslow RA. Poor interobserver reproducibility in the diagnosis of high-grade endometrial carcinoma. Am J Surg Pathol. 2013; 37:874–881.31. Hamilton CA, Cheung MK, Osann K, Chen L, Teng NN, Longacre TA, et al. Uterine papillary serous and clear cell carcinomas predict for poorer survival compared to grade 3 endometrioid corpus cancers. Br J Cancer. 2006; 94:642–646.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of minimal uterine serous carcinoma with distant lymph node metastasis without peritoneal dissemination
- Para-aortic Lymph Node Dissection in Gastric Cancer
- Prognosis of the Patients Showing Metastasis to the Para-aortic or/and Supraclavicular Lymph Nodes at the Time of Diagnosis of Recurrence of the Cervical Cancer
- Laparoscopic Para-aortic Lymph Node Dissection in Patients with Gynecologic Malignancy
- An ovarian mucinous cystadenocarcinoma arising from mature cystic teratoma with para-aortic lymph node metastasis: a case report