Yonsei Med J.
2008 Oct;49(5):792-803. 10.3349/ymj.2008.49.5.792.
Association between Fecal Bile Acids and Colorectal Cancer: A Meta-analysis of Observational Studies
- Affiliations
-
- 1Department of Gastroenterology, Ren Ji Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai Institute of Digestive Disease, Shanghai, China. z-ran@online.sh.cn
- 2Department of Immunology, Shanxi Medical University, Taiyuan, China.
- KMID: 2158198
- DOI: http://doi.org/10.3349/ymj.2008.49.5.792
Abstract
- PURPOSE
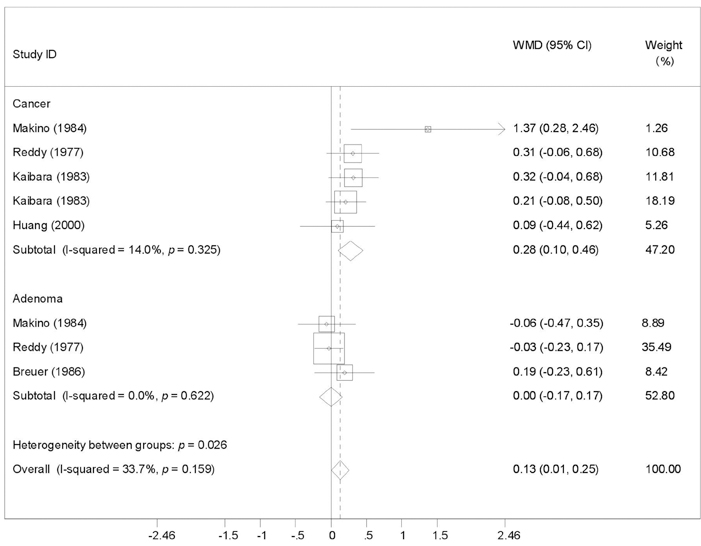
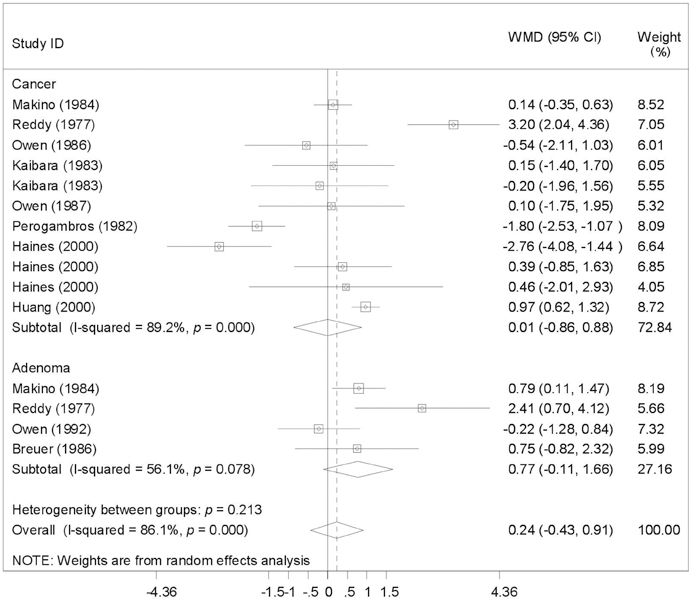
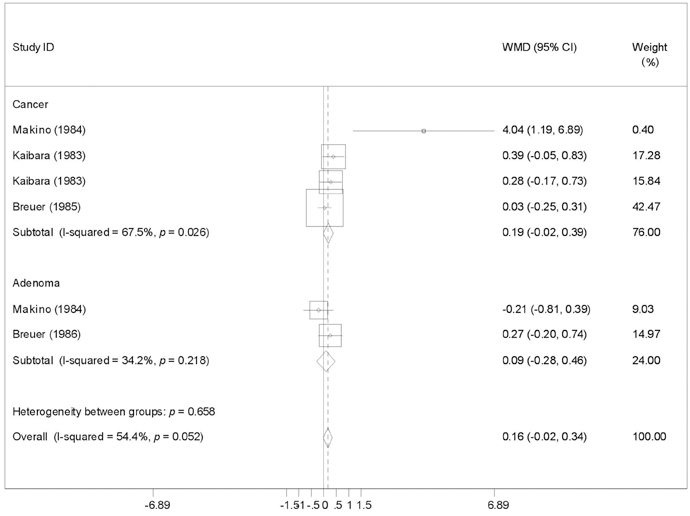
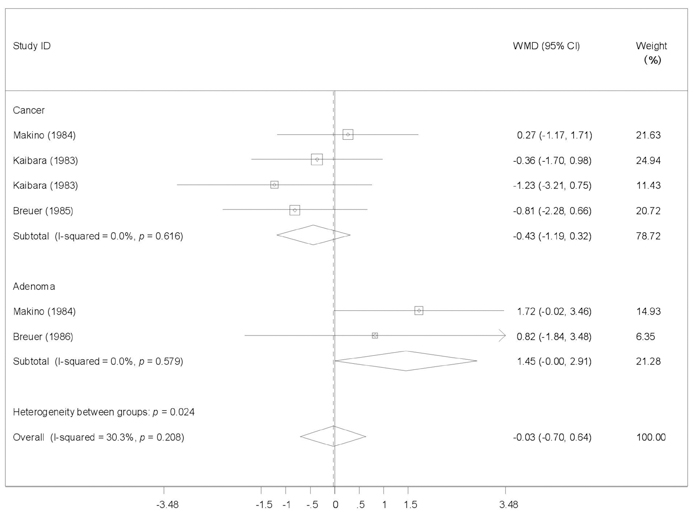
To provide a systematic review with meta-analysis for addressing the relationship between fecal bile acids (FBAs) and colorectal cancer. MATERIALS AND METHODS: Electronic databases were searched for all observational studies that examined the relationship between FBAs and colorectal cancer or adenoma, and calculated weighted mean difference (WMD) and 95% confidence interval (CI). Publication bias was assessed with funnel plot. RESULTS: Twenty case-control or cohort studies were identified. All studies were pooled to assess the relationship between total FBAs and cancer/adenoma of the large bowel, however, no association was seen (WMD 0.61mg/g freeze-dried feces; 95% CI: -0.35-1.57). Significantly increased concentration of chenodeoxycholic acid (CDCA) was seen while pooling to assess the relationship between CDCA and cancer/adenoma of the large bowel (WMD 0.13 mg/g freeze-dried feces; 95% CI: 0.01-0.25), especially for colorectal cancer (WMD 0.28mg/g freeze-dried feces; 95% CI: 0.10-0.46). However, no significant differences in deoxycholic acid (DCA), lithocholic acid (LCA), and primary and secondary bile acids, were seen between patients with cancer and patients with matched controls regardless of fixed and random effects models. CONCLUSION: CDCA might play a role in the etiology of colorectal cancer.
Keyword
MeSH Terms
Figure
Reference
-
1. Hill MJ, Drasar BS, Williams RE, Meade TW, Cox AG, Simpson JE, et al. Faecal bile-acids and clostridia in patients with cancer of the large bowel. Lancet. 1975. 1:535–539.2. Bayerdörffer E, Mannes GA, Richter WO, Ochsenkühn T, Wiebecke B, Köpcke W, et al. Increased serum deoxycholic acid levels in men with colorectal adenomas. Gastroenterology. 1993. 104:145–151.
Article3. Debruyne PR, Bruyneel EA, Karaguni IM, Li X, Flatau G, Müller O, et al. Bile acids stimulate invasion and haptotaxis in human colorectal cancer cells through activation of multiple oncogenic signaling pathways. Oncogene. 2002. 21:6740–6750.
Article4. Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990. 61:759–767.
Article5. Reddy BS, Wynder EL. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977. 39:2533–2539.
Article6. Mudd DG, McKelvey ST, Norwood W, Elmore DT. Carcinoma of the large bowel and faecal bile acids. Br J Surg. 1979. 66:355.7. Murray WR, Backwood A, Trotter JM, Calman KC, MacKay C. Faecal bile acids and clostridia in the aetiology of colorectal cancer. Br J Cancer. 1980. 41:923–928.
Article8. Morson B. President's address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 1974. 67:451–457.9. Mudd DG, McKelvey ST, Norwood W, Elmore DT, Roy AD. Faecal bile acid concentration of patients with carcinoma or increased risk of carcinoma in the large bowel. Gut. 1980. 21:587–590.
Article10. Hikasa Y, Tanida N, Ohno T, Shimoyama T. Faecal bile acid profiles in patients with large bowel cancer in Japan. Gut. 1984. 25:833–838.
Article11. Makino T. Fecal bile acid excretion in patients with colon cancer, colon polyp and peptic ulcer. Tokai J Exp Clin Med. 1984. 9:297–305.12. Owen RW, Henly PJ, Thompson MH, Hill MJ. Steroids and cancer: faecal bile acid screening for early detection of cancer risk. J Steroid Biochem. 1986. 24:391–394.
Article13. Kaibara N, Sasaki T, Ikeguchi M, Koga S, Ikawa S. Fecal bile acids and neutral sterols in Japanese with large bowel carcinoma. Oncology. 1983. 40:255–258.
Article14. Owen RW, Dodo M, Thompson MH, Hill MJ. Fecal steroids and colorectal cancer. Nutr Cancer. 1987. 9:73–80.15. Murray WR, Backwood A, Trotter JM, Calman KC, MacKay C. Faecal bile acids and clostridia in the aetiology of colorectal cancer. Br J Cancer. 1980. 41:923–928.16. Korpela JT, Adlercreutz H, Turunen MJ. Fecal free and conjugated bile acids and neutral sterols in vegetarians, omnivores, and patients with colorectal cancer. Scand J Gastroenterol. 1988. 23:277–283.
Article17. Meance S, Boutron-Ruault MC, Myara A, Gerhardt MF, Marteau P, Lavergne A, et al. Fecal primary bile acids and serum cholesterol are associated with colorectal adenomas. Dig Dis Sci. 2003. 48:1751–1757.18. Owen RW, Day DW, Thompson MH. Faecal steroids and colorectal cancer: steroid profiles in subjects with adenomatous polyps of the large bowel. Eur J Cancer Prev. 1992. 1:105–112.19. Breuer NF, Jaekel S, Dommes P, Goebell H. Fecal bile acids in patients with adenomatous polyps of the colon. Case-control study. Digestion. 1986. 34:87–92.
Article20. Haines A, Hill MJ, Thompson MH, Owen RW, Williams RE, Meade TW, et al. A prospective study of faecal bile acids and colorectal cancer. Eur J Cancer Prev. 2000. 9:317–323.
Article21. Hill MJ, Melville DM, Lennard-Jones JE, Neale K, Ritchie JK. Faecal bile acids, dysplasia, and carcinoma in ulcerative colitis. Lancet. 1987. 2:185–186.22. Perogambros A, Legakis NJ. Faecal bile acids in patients with colon cancer. Zentralbl Bakteriol Mikrobiol Hyg [B]. 1982. 176:346–348.23. Breuer NF, Dommes P, Jaekel S, Goebell H. Fecal bile acid excretion pattern in colonic cancer patients. Dig Dis Sci. 1985. 30:852–859.
Article24. Tanida N, Hikasa Y, Shimoyama T, Setchell KD. Comparison of faecal bile acid profiles between patients with adenomatous polyps of the large bowel and healthy subjects in Japan. Gut. 1984. 25:824–832.25. Huang JG, Yu JP, Shen ZX. The metabolism feature of fecal bile acids in patients with colorectal cancer. Anthology Med. 2000. 19:429–431.26. Boutron-Ruault MC, Marteau P, Lavergne-Slove A, Myara A, Gerhardt MF, Franchisseur C, et al. Effects of a 3-mo consumption of short-chain fructo-oligosaccharides on parameters of colorectal carcinogenesis in patients with or without small or large colorectal adenomas. Nutr Cancer. 2005. 53:160–168.
Article27. American Cancer Society. Colorectal Cancer Facts & Figures. Statistics for 2005. Accessed June 14, 2005. Available at: http://www.cancer.org/downloads/STT/CAFF2005CR4PWSecured.pdf.28. Sandler RS. Epidemiology and risk factors for colorectal cancer. Gastroenterol Clin North Am. 1996. 25:717–735.
Article29. Soma T, Kaganoi J, Kawabe A, Kondo K, Tsunoda S, Imamura M, et al. Chenodeoxycholic acid stimulates the progression of human esophageal cancer cells: A possible mechanism of angiogenesis in patients with esophageal cancer. Int J Cancer. 2006. 119:771–782.30. Reddy BS, Mastromarino A, Wynder EL. Further leads on metabolic epidemiology of large bowel cancer. Cancer Res. 1975. 35:3403–3406.31. Narisawa T, Magadia NE, Weisburger JH, Wynder EL. Promoting effect of bile acids on colon carcinogenesis after intrarectal instillation of N-methyl-N'-nitro-N-nitrosoguanidine in rats. J Natl Cancer Inst. 1974. 53:1093–1097.32. Kishida T, Taguchi F, Feng L, Tatsuguchi A, Sato J, Fujimori S, et al. Analysis of bile acids in colon residual liquid or fecal material in patients with colorectal neoplasia and control subjects. J Gastroenterol. 1997. 32:306–311.
Article33. Ayaki Y, Tsuma-Date T, Endo S, Ogura M. Role of endogenous and exogenous cholesterol in liver as the precursor for bile acids in rats. Steroids. 1981. 38:495–509.34. Gustafsson BE, Angelin B, Einarsson K, Gustafsson JA. Effects of cholesterol feeding on synthesis and metabolism of cholesterol and bile acids in germfree rats. J Lipid Res. 1977. 18:717–721.35. Reddy BS, Weisburger JH, Wynder EL. Effects of high risk and low risk diets for colon carcinogenesis on fecal microflora and steroids in man. J Nutr. 1975. 105:878–884.
Article36. Reddy BS, Wynder EL. Large-bowel carcinogenesis: fecal constituents of populations with diverse incidence rates of colon cancer. J Natl Cancer Inst. 1973. 50:1437–1442.
Article37. Kamano T, Mikami Y, Kurasawa T, Tsurumaru M, Matsumoto M, Kano M, et al. Ratio of primary and secondary bile acids in feces: possible marker for colorectal cancer? Dis Colon Rectum. 1999. 42:668–672.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dietary Intake of Omega-3 fatty acids and Endocrine-related Gynecological Cancer: A Meta-Analysis of Observational Studies
- The Analysis of Gallbladder Bile Acid Concentration in Patients with Colorectal Cancer
- High pressure liquid chromatographic analysis of conjugated bile acids in gallbladder bile of Korean adults
- Effect of Bile Acids on Biliary Excretion of Cholesterol in Rabbits
- Biliary Bile Acid Analysis in the Patients with Bile Duct Cancer