Yonsei Med J.
2008 Oct;49(5):714-718. 10.3349/ymj.2008.49.5.714.
Low-dose Methotrexate Therapy for Intravenous Immunoglobulin-resistant Kawasaki Disease
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Pochon CHA University, Seongnam, Korea. dskim6634@yuhs.ac
- 2Department of Pediatrics, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2158188
- DOI: http://doi.org/10.3349/ymj.2008.49.5.714
Abstract
-
PURPOSE: The aim of this study was to evaluate the efficacy of low-dose oral methotrexate (MTX) as a treatment for patients with Kawasaki disease (KD) which was resistant to intravenous immunoglobulin (IVIG).
PATIENTS AND METHODS
The patients who had persistent or recrudescent fever after treatment with IVIG were subsequently treated with low-dose oral MTX [10mg/body surface area (BSA)] once weekly.
RESULTS
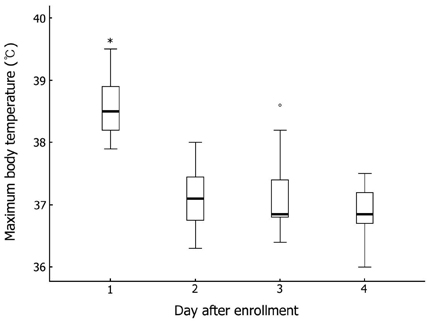
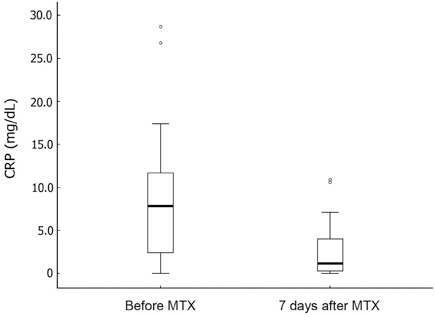
Seventeen patients developed persistent or recrudescent fever after treatment of KD with IVIG and were consequently given MTX. The proportion of children with coronary artery lesions (CALs) was 76%. The median value of maximum body temperatures decreased significantly within 24 hours of MTX therapy (38.6degrees C vs. 37.0degrees C, p < 0.001). The median CRP (C-reactive protein) level was found to be significantly lower 1 week after administering the first dose of MTX (8.9mg/dL vs. 1.2mg/dL, p < 0.001). The median duration of fever before MTX treatment was shorter in CALs (-) group than in CALs (+) group (7 days vs. 10 days, p = 0.023). No adverse effects of MTX were observed.
CONCLUSION
MTX treatment for IVIG-resistant KD resulted in quick resolution of fever and rapid improvement of inflammation markers without causing any adverse effects. MTX therapy should further be assessed in a multicenter, placebo-blinded trial to evaluate whether it also improves coronary artery outcome.
MeSH Terms
Figure
Cited by 3 articles
-
Immunoglobulin VH Chain Gene Analysis of Peripheral Blood IgM-Producing B Cells in Patients with Kawasaki Disease
Hyun Hee Lee, Jun Soo Shin, Dong Soo Kim
Yonsei Med J. 2009;50(4):493-504. doi: 10.3349/ymj.2009.50.4.493.Kawasaki Disease: Laboratory Findings and an Immunopathogenesis on the Premise of a "Protein Homeostasis System"
Kyung-Yil Lee, Jung-Woo Rhim, Jin-Han Kang
Yonsei Med J. 2012;53(2):262-275. doi: 10.3349/ymj.2012.53.2.262.Clinical Outcomes of Low-Dose Methotrexate Therapy as a Second-Line Drug for Intravenous Immunoglobulin-Resistant Kawasaki Disease
Hyejin Jang, Kyu Yeun Kim, Dong Soo Kim
Yonsei Med J. 2018;59(1):113-118. doi: 10.3349/ymj.2018.59.1.113.
Reference
-
1. Furusho K, Kamiya T, Nakano H, Kiyosawa N, Shinomiya K, Hayashidera T, et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet. 1984. 2:1055–1058.
Article2. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986. 315:341–347.
Article3. Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re-treatment in Kawasaki disease. J Pediatr. 1993. 123:657–659.
Article4. Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. US/Canadian Kawasaki Syndrome Study Group. Pediatr Infect Dis J. 1998. 17:1144–1148.
Article5. Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin-resistant Kawasaki disease with pulsed doses of corticosteroids. J Pediatr. 1996. 128:146–149.
Article6. Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001. 43:211–217.
Article7. Lee MS, An SY, Jang GC, Kim DS. A case of intravenous immunoglobulin-resistant Kawasaki disease treated with methotrexate. Yonsei Med J. 2002. 43:527–532.
Article8. Ahn SY, Kim DS. Treatment of intravenous immunoglobulin-resistant Kawasaki disease with methotrexate. Scand J Rheumatol. 2005. 34:136–139.9. Han BK, Lee HS, Kil HR, Han HS, Lee JH, Chung YH. A study on clinical application of Harada's scoring method to Kawasaki disease: suggesting the revision of the criteria for IVIG treatment of KD in Korea. J Korean Pediatr Soc. 1997. 40:539–548.10. Research Committee on Kawasaki Disease. Report of subcommittee on standardization of diagnostic criteria and reporting of coronary artery lesions in Kawasaki disease. 1984. Tokyo, Japan: Ministry of Health and Welfare.11. Figueíredo F, Uhing RJ, Okonogi K, Gettys TW, Johnson SP, Adams DO, et al. Activation of the cAMP cascade inhibits an early event involved in murine macrophage Ia expression. J Biol Chem. 1990. 265:12317–12323.
Article12. Snijdewint FG, Kalinski P, Wierenga EA, Bos JD, Kapsenberg ML. Prostaglandin E2 differentially modulates cytokine secretion profiles of human T helper lymphocytes. J Immunol. 1993. 150:5321–5329.13. Riksen NP, Barrera P, van den Broek PH, van Riel PL, Smits P, Rongen GA. Methotrexate modulates the kinetics of adenosine in humans in vivo. Ann Rheum Dis. 2006. 65:465–470.14. Furukawa S, Matsubara T, Jujoh K, Yone K, Sugawara T, Sasai K, et al. Peripheral blood monocyte/macrophages and serum tumor necrosis factor in Kawasaki disease. Clin Immunol Immunopathol. 1988. 48:247–251.
Article15. Maury CP, Salo E, Pelkonen P. Circulating interleukin-1 beta in patients with Kawasaki disease. N Engl J Med. 1988. 319:1670–1671.16. Rooney TW, Furst DE, Koehnke R, Burmeister L. Aspirin is not associated with more toxicity than other nonsteroidal antiinflammatory drugs in patients with rheumatoid arthritis treated with methotrexate. J Rheumatol. 1993. 20:1297–1302.17. Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000. 105:E78.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Outcomes of Low-Dose Methotrexate Therapy as a Second-Line Drug for Intravenous Immunoglobulin-Resistant Kawasaki Disease
- Predictors and management of intravenous immunoglobulin-resistant Kawasaki disease
- Efficacy of Dexamethasone Therapy for Coronary Lesion after Immunoglobulin-retreated Kawasaki Disease
- A Case of Intravenous Immunoglobulin-Resistant Kawasaki Disease Treated with Methotrexate
- Treatment of Intravenous Immune-Globulin Resistant Kawasaki Disease with Corticosteroids