The Prevalence, Genotype and Antimicrobial Susceptibility of High- and Low-Level Mupirocin Resistant Methicillin-Resistant Staphylococcus aureus
- Affiliations
-
- 1Department of Dermatology, Wonkwang University School of Medicine, Iksan, Korea. sdpark@wonkwang.ac.kr
- 2Department of Clinical Laboratory Science, Wonkwang Health Science University, Iksan, Korea.
- 3Institute of Wonkwang Medical Science, Wonkwang University School of Medicine, Iksan, Korea.
- KMID: 2156853
- DOI: http://doi.org/10.5021/ad.2012.24.1.32
Abstract
- BACKGROUND
Mupirocin has been used for the treatment of skin infections and eradication of nasal carriage of methicillin-resistant Staphylococcus aureus (MRSA). The increased use of this antibiotic has been accompanied by outbreaks of MRSA that are resistant to mupirocin.
OBJECTIVE
This study aims to determine the prevalence, genotype and antimicrobial susceptibility of mupirocin-resistant MRSA from 4 Korean hospitals.
METHODS
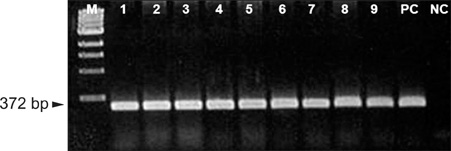
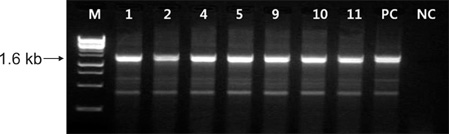
A total 193 MRSA clinical isolates were collected from four university hospitals. Antimicrobial susceptibility tests, including mupirocin, and pulsed-field gel electrophoresis (PFGE) pattern analysis were performed.
RESULTS
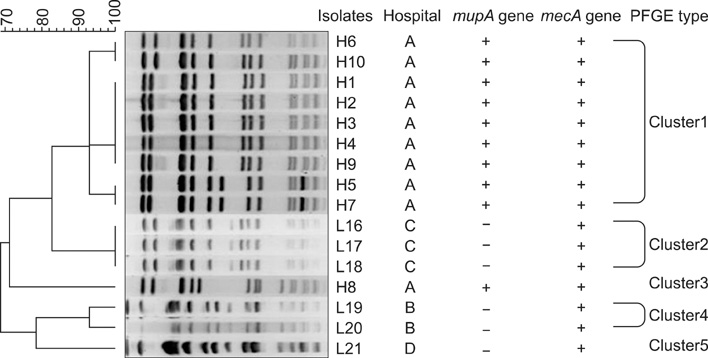
Overall, 27 of the 193 (14.1%) MRSA isolates were resistant to mupirocin. All of the (A) hospital isolates showed high-level (HL) mupirocin resistance and the low-level (LL) mupirocin resistant strains were from three other hospitals. The PFGE patterns of 16 mupirocin-resistant isolates were divided into 5 clusters (1-5), and the nine HL mupirocin-resistant isolates belonged to cluster 1. Both the HL and LL mupirocin-resistant MRSA isolates were susceptible to vancomycin and rifampin, but they were resistant to ciprofloxacin, clindamycin and tetracycline. The erythromycin and fusidic acid resistance rates were different between the HL and LL resistant isolates.
CONCLUSION
HL mupirocin-resistant isolates that could transfer this resistance to other bacteria were detected and these isolates were clonally related. The emergence of mupirocin resistant isolates emphasizes the importance of using antibiotics judiciously and carefully monitoring the prevalence of mupirocin resistance.
MeSH Terms
-
Anti-Bacterial Agents
Bacteria
Ciprofloxacin
Clindamycin
Disease Outbreaks
Electrophoresis, Gel, Pulsed-Field
Erythromycin
Fusidic Acid
Genotype
Hospitals, University
Methicillin Resistance
Methicillin-Resistant Staphylococcus aureus
Mupirocin
Prevalence
Rifampin
Skin
Tetracycline
Vancomycin
Anti-Bacterial Agents
Ciprofloxacin
Clindamycin
Erythromycin
Fusidic Acid
Mupirocin
Rifampin
Tetracycline
Vancomycin
Figure
Cited by 4 articles
-
Antibiotic Susceptibility of Staphylococcus aureus in Atopic Dermatitis: Current Prevalence of Methicillin-Resistant Staphylococcus aureus in Korea and Treatment Strategies
Mi-Young Jung, Jong-Youn Chung, Hae-Young Lee, Jiho Park, Dong-Youn Lee, Jun-Mo Yang
Ann Dermatol. 2015;27(4):398-403. doi: 10.5021/ad.2015.27.4.398.In Vitro Antimicrobial Activities of Fusidic Acid and Retapamulin against Mupirocin- and Methicillin-Resistant Staphylococcus aureus
Sang Hyun Park, Jin Kyung Kim, Kun Park
Ann Dermatol. 2015;27(5):551-556. doi: 10.5021/ad.2015.27.5.551.Change in Antimicrobial Susceptibility of Skin-Colonizing Staphylococcus aureus in Korean Patients with Atopic Dermatitis during Ten-Year Period
Jung-Min Park, Ju-Hyun Jo, Hyunju Jin, Hyun-Chang Ko, Moon-Bum Kim, Jung-Min Kim, Do-Won Kim, Ho-Sun Jang, Byung-Soo Kim
Ann Dermatol. 2016;28(4):470-478. doi: 10.5021/ad.2016.28.4.470.Colonization of Staphylococcus aureus and sensitivity to antibiotics in children with atopic dermatitis
Yoonha Hwang, Joon Seok Kang, Byoung Kuk Kim, Sung Won Kim
Allergy Asthma Respir Dis. 2017;5(1):21-26. doi: 10.4168/aard.2017.5.1.21.
Reference
-
1. Sutherland R, Boon RJ, Griffin KE, Masters PJ, Slocombe B, White AR. Antibacterial activity of mupirocin (pseudomonic acid), a new antibiotic for topical use. Antimicrob Agents Chemother. 1985. 27:495–498.
Article2. Simor AE, Stuart TL, Louie L, Watt C, Ofner-Agostini M, Gravel D, et al. Canadian Nosocomial Infection Surveillance Program. Mupirocin-resistant, methicillin-resistant Staphylococcus aureus strains in Canadian hospitals. Antimicrob Agents Chemother. 2007. 51:3880–3886.
Article3. Cookson BD. The emergence of mupirocin resistance: a challenge to infection control and antibiotic prescribing practice. J Antimicrob Chemother. 1998. 41:11–18.
Article4. Yun HJ, Lee SW, Yoon GM, Kim SY, Choi S, Lee YS, et al. Prevalence and mechanisms of low- and high-level mupirocin resistance in staphylococci isolated from a Korean hospital. J Antimicrob Chemother. 2003. 51:619–623.
Article5. Yoo JI, Shin ES, Cha JO, Lee JK, Jung YH, Lee KM, et al. Clonal dissemination and mupA gene polymorphism of mupirocin-resistant Staphylococcus aureus isolates from long-term-care facilities in South Korea. Antimicrob Agents Chemother. 2006. 50:365–367.
Article6. Yang JA, Park DW, Sohn JW, Yang IS, Kim KH, Kim MJ. Molecular analysis of isoleucyl-tRNA synthetase mutations in clinical isolates of methicillin-resistant Staphylococcus aureus with low-level mupirocin resistance. J Korean Med Sci. 2006. 21:827–832.
Article7. Clinical and Laboratory Standards Institute. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard. 2009. eighth edition. Wayne: Clinical and Laboratory Standards Institute.
Article8. Lee HK, Lee EJ, Pahk YJ, Kim BK, Kang MW, Shim SI. Relationship between the level of methicillin resistance and mecA, mecI, femA genes genes in Staphylococci. Korean J Infect Dis. 1998. 30:36–44.
Article9. Yokoyama T. Study on mec gene in methicillin-resistant staphylococci. Kansenshogaku Zasshi. 1993. 67:1203–1210.
Article10. Ramsey MA, Bradley SF, Kauffman CA, Morton TM. Identification of chromosomal location of mupA gene, encoding low-level mupirocin resistance in staphylococcal isolates. Antimicrob Agents Chemother. 1996. 40:2820–2823.
Article11. Murchan S, Kaufmann ME, Deplano A, de Ryck R, Struelens M, Zinn CE, et al. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J Clin Microbiol. 2003. 41:1574–1585.12. Heyndrickx M, Vandemeulebroecke K, Scheldeman P, Kersters K, de Vos P, Logan NA, et al. A polyphasic reassessment of the genus Paenibacillus, reclassification of Bacillus lautus (Nakamura 1984) as Paenibacillus lautus comb. nov., and of Bacillus peoriae (Montefusco et al. 1993) as Paenibacillus peoriae comb. nov., and emended descriptions of P. lautus and of P. peoriae. Int J Syst Bacteriol. 1996. 46:988–1003.13. Sokal RR, Michener CD. A statistical method for evaluating systematic relationships. University of Kansas Scientific Bulletin. 1958. 28:1409–1438.
Article14. Rahman M, Noble WC, Cookson B, Baird D, Coia J. Mupirocinresistant staphylococcus aureus. Lancet. 1987. 330:387–388.
Article15. Conly JM, Vas S. Increasing mupirocin resistance of Staphylococcus aureus in CAPD--should it continue to be used as prophylaxis? Perit Dial Int. 2002. 22:649–652.
Article16. Pérez-Fontán M, Rosales M, Rodríguez-Carmona A, Falcón TG, Valdés F. Mupirocin resistance after long-term use for Staphylococcus aureus colonization in patients undergoing chronic peritoneal dialysis. Am J Kidney Dis. 2002. 39:337–341.
Article17. Wise R, Johnson J. Mupirocin resistance. Lancet. 1991. 338(8766):578.
Article18. Lee HJ, Suh JT, Kim YS, Lenz W, Bierbaum G, Schaal KP. Typing and antimicrobial susceptibilities of methicillin resistant Staphylococcus aureus (MRSA) strains isolated in a hospital in Korea. J Korean Med Sci. 2001. 16:381–385.
Article19. Redhead RJ, Lamb YJ, Rowsell RB. The efficacy of calcium mupirocin in the eradication of nasal Staphylococcus aureus carriage. Br J Clin Pract. 1991. 45:252–254.
Article20. Janssen DA, Zarins LT, Schaberg DR, Bradley SF, Terpenning MS, Kauffman CA. Detection and characterization of mupirocin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993. 37:2003–2006.
Article21. Chaves F, García-Martínez J, de Miguel S, Otero JR. Molecular characterization of resistance to mupirocin in methicillin-susceptible and -resistant isolates of Staphylococcus aureus from nasal samples. J Clin Microbiol. 2004. 42:822–824.
Article22. Thomas DG, Wilson JM, Day MJ, Russell AD. Mupirocin resistance in staphylococci: development and transfer of isoleucyl-tRNA synthetase-mediated resistance in vitro. J Appl Microbiol. 1999. 86:715–722.
Article23. Bastos MC, Mondino PJ, Azevedo ML, Santos KR, Giambiagi-deMarval M. Molecular characterization and transfer among Staphylococcus strains of a plasmid conferring high-level resistance to mupirocin. Eur J Clin Microbiol Infect Dis. 1999. 18:393–398.
Article24. Kim SM, Lee DC, Park SD, Kim BS, Kim JK, Choi MR, et al. Genotype, coagulase type and antimicrobial susceptibility of methicillin-resistant staphylococcus aureus isolated from dermatology patients and Healthy Individuals in Korea. J Bacteriol Virol. 2009. 39:307–316.
Article25. Maple PA, Hamilton-Miller JM, Brumfitt W. Comparison of the in-vitro activities of the topical antimicrobials azelaic acid, nitrofurazone, silver sulphadiazine and mupirocin against methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 1992. 29:661–668.
Article26. Riley TV, Carson CF, Bowman RA, Mulgrave L, Golledge CL, Pearman JW, et al. Mupirocin-resistant methicillin-resistant Staphylococcus aureus in Western Australia. Med J Aust. 1994. 161:397–398.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevalence and Clinical Characteristics of Mupirocin-Resistant Staphylococcus aureus
- In Vitro Antimicrobial Activities of Fusidic Acid and Retapamulin against Mupirocin- and Methicillin-Resistant Staphylococcus aureus
- Isolation and Antimicrobial Susceptibility of Mupirocin-Resistant and Methicillin-Resistant Staphylococcus aureus from Clinical Samples
- Antibiotic Susceptibility of Staphylococcus aureus in Atopic Dermatitis: Current Prevalence of Methicillin-Resistant Staphylococcus aureus in Korea and Treatment Strategies
- Methicillin-resistant Staphylococcus aureus (MRSA) Infection in Neonates