Ann Dermatol.
2012 Feb;24(1):1-6. 10.5021/ad.2012.24.1.1.
Topical Hypopigmenting Agents for Pigmentary Disorders and Their Mechanisms of Action
- Affiliations
-
- 1Department of Dermatology, Seoul National University Bundang Hospital, Seongnam, Korea. gcpark@snu.ac.kr
- 2Department of Biochemistry, Chung-Ang University College of Medicine, Seoul, Korea.
- KMID: 2156847
- DOI: http://doi.org/10.5021/ad.2012.24.1.1
Abstract
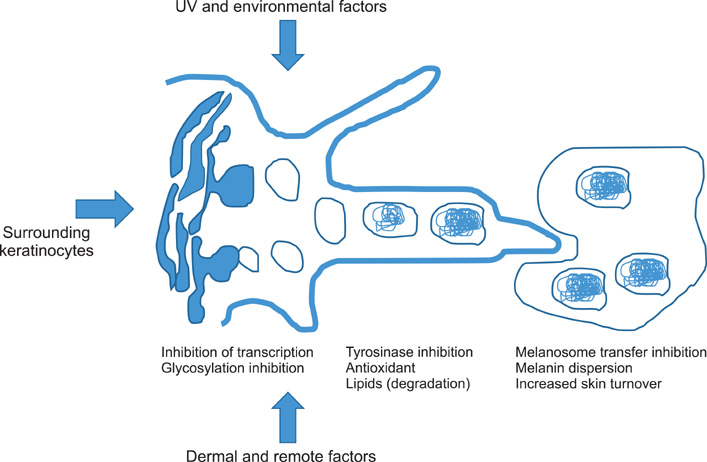
- Melanin is produced in melanocytes and stored in melanosomes. In spite of its beneficial sun-protective effect, abnormal accumulation of melanin results in esthetic problems. Hydroquinone, competing with tyrosine, is a major ingredient in topical pharmacological agents. However, frequent adverse reactions are amongst its major limitation. To solve this problem, several alternatives such as arbutin, kojic acid, aloesin, and 4-n-butyl resorcinol have been developed. Herein, we classify hypopigmenting agents according to their mechanism of action; a) regulation of enzyme, which is subdivided into three categories, i) regulation of transcription and maturation of tyrosinase, ii) inhibition of tyrosinase activity, and iii) post-transcriptional control of tyrosinase; b) inhibition of melanosome transfer, and c) additional mechanisms such as regulation of the melanocyte environment and antioxidant agents.
Keyword
MeSH Terms
Figure
Reference
-
1. Gupta AK, Gover MD, Nouri K, Taylor S. The treatment of melasma: a review of clinical trials. J Am Acad Dermatol. 2006. 55:1048–1065.
Article2. Amer M, Metwalli M. Topical hydroquinone in the treatment of some hyperpigmentary disorders. Int J Dermatol. 1998. 37:449–450.
Article3. Rendon MI. Utilizing combination therapy to optimize melasma outcomes. J Drugs Dermatol. 2004. 3:5 Suppl. S27–S34.4. Bolognia JL, Sodi SA, Osber MP, Pawelek JM. Enhancement of the depigmenting effect of hydroquinone by cystamine and buthionine sulfoximine. Br J Dermatol. 1995. 133:349–357.
Article5. Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003. 16:101–110.
Article6. Kang HY, Valerio L, Bahadoran P, Ortonne JP. The role of topical retinoids in the treatment of pigmentary disorders: an evidence-based review. Am J Clin Dermatol. 2009. 10:251–260.7. Kim DS, Kim HJ, Choi KH, Chung JH, Kim KH, Par KC. UVB-induced GM-CSF production is suppressed by dexamethasone in HaCaT cells. Photodermatol Photoimmunol Photomed. 2001. 17:121–125.
Article8. Lee IW, Lee SC, Kim DS, Kim HJ, Park KC. Effects of dexamethasone on endothelin-1 (ET-1) production by keratinocytes. Ann Dermatol. 2001. 13:148–152.
Article9. Torok HM. A comprehensive review of the long-term and short-term treatment of melasma with a triple combination cream. Am J Clin Dermatol. 2006. 7:223–230.
Article10. Taylor SC, Torok H, Jones T, Lowe N, Rich P, Tschen E, et al. Efficacy and safety of a new triple-combination agent for the treatment of facial melasma. Cutis. 2003. 72:67–72.11. Grimes P, Kelly AP, Torok H, Willis I. Community-based trial of a triple-combination agent for the treatment of facial melasma. Cutis. 2006. 77:177–184.12. Torok HM, Jones T, Rich P, Smith S, Tschen E. Hydroquinone 4%, tretinoin 0.05%, fluocinolone acetonide 0.01%: a safe and efficacious 12-month treatment for melasma. Cutis. 2005. 75:57–62.13. Hexsel D, Arellano I, Rendon M. Ethnic considerations in the treatment of Hispanic and Latin-American patients with hyperpigmentation. Br J Dermatol. 2006. 156:Suppl 1. 7–12.
Article14. Chan R, Park KC, Lee MH, Lee ES, Chang SE, Leow YH, et al. A randomized controlled trial of the efficacy and safety of a fixed triple combination (fluocinolone acetonide 0.01%, hydroquinone 4%, tretinoin 0.05%) compared with hydroquinone 4% cream in Asian patients with moderate to severe melasma. Br J Dermatol. 2008. 159:697–703.
Article15. Yasumoto K, Yokoyama K, Takahashi K, Tomita Y, Shibahara S. Functional analysis of microphthalmia-associated transcription factor in pigment cell-specific transcription of the human tyrosinase family genes. J Biol Chem. 1997. 272:503–509.
Article16. Kim DS, Hwang ES, Lee JE, Kim SY, Kwon SB, Park KC. Sphingosine-1-phosphate decreases melanin synthesis via sustained ERK activation and subsequent MITF degradation. J Cell Sci. 2003. 116:1699–1706.
Article17. Kim DS, Park SH, Park KC. Transforming growth factor-beta1 decreases melanin synthesis via delayed extracellular signal-regulated kinase activation. Int J Biochem Cell Biol. 2004. 36:1482–1491.
Article18. Kim DS, Park SH, Kwon SB, Youn SW, Park KC. Effects of lysophosphatidic acid on melanogenesis. Chem Phys Lipids. 2004. 127:199–206.
Article19. Kim DS, Kim SY, Moon SJ, Chung JH, Kim KH, Cho KH, et al. Ceramide inhibits cell proliferation through Akt/PKB inactivation and decreases melanin synthesis in Mel-Ab cells. Pigment Cell Res. 2001. 14:110–115.
Article20. Kim DS, Kim SY, Chung JH, Kim KH, Eun HC, Park KC. Delayed ERK activation by ceramide reduces melanin synthesis in human melanocytes. Cell Signal. 2002. 14:779–785.
Article21. Mishima Y, Imokawa G. Selective aberration and pigment loss in melanosomes of malignant melanoma cells in vitro by glycosylation inhibitors: premelanosomes as glycoprotein. J Invest Dermatol. 1983. 81:106–114.
Article22. Franchi J, Coutadeur MC, Marteau C, Mersel M, Kupferberg A. Depigmenting effects of calcium D-pantetheine-S-sulfonate on human melanocytes. Pigment Cell Res. 2000. 13:165–171.
Article23. Sugai T. Clinical effects of arbutin in patients with chloasma (in Japanese). Hifu (Skin Res). 1992. 34:522–529.24. Chakraborty AK, Funasaka Y, Komoto M, Ichihashi M. Effect of arbutin on melanogenic proteins in human melanocytes. Pigment Cell Res. 1998. 11:206–212.
Article25. Moon KY, Ahn KS, Lee J, Kim YS. Kojic acid, a potential inhibitor of NF-kappaB activation in transfectant human HaCaT and SCC-13 cells. Arch Pharm Res. 2001. 24:307–311.
Article26. Kim DS, Kim SY, Park SH, Choi YG, Kwon SB, Kim MK, et al. Inhibitory effects of 4-n-butylresorcinol on tyrosinase activity and melanin synthesis. Biol Pharm Bull. 2005. 28:2216–2219.
Article27. Huh SY, Shin JW, Na JI, Huh CH, Youn SW, Park KC. Efficacy and safety of liposome-encapsulated 4-n-butylresorcinol 0.1% cream for the treatment of melasma: a randomized controlled split-face trial. J Dermatol. 2010. 37:311–315.
Article28. Fenoll LG, Rodríguez-López JN, Varón R, García-Ruiz PA, García-Cánovas F, Tudela J. Action mechanism of tyrosinase on meta- and para-hydroxylated monophenols. Biol Chem. 2000. 381:313–320.
Article29. Ando H, Ryu A, Hashimoto A, Oka M, Ichihashi M. Linoleic acid and alpha-linolenic acid lightens ultraviolet-induced hyperpigmentation of the skin. Arch Dermatol Res. 1998. 290:375–381.
Article30. Ando H, Funasaka Y, Oka M, Ohashi A, Furumura M, Matsunaga J, et al. Possible involvement of proteolytic degradation of tyrosinase in the regulatory effect of fatty acids on melanogenesis. J Lipid Res. 1999. 40:1312–1316.
Article31. Ando H, Wen ZM, Kim HY, Valencia JC, Costin GE, Watabe H, et al. Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway. Biochem J. 2006. 394:43–50.
Article32. Ando H, Watabe H, Valencia JC, Yasumoto K, Furumura M, Funasaka Y, et al. Fatty acids regulate pigmentation via proteasomal degradation of tyrosinase: a new aspect of ubiquitin-proteasome function. J Biol Chem. 2004. 279:15427–15433.
Article33. Martínez-Esparza M, Ferrer C, Castells MT, García-Borrón JC, Zuasti A. Transforming growth factor beta1 mediates hypopigmentation of B16 mouse melanoma cells by inhibition of melanin formation and melanosome maturation. Int J Biochem Cell Biol. 2001. 33:971–983.
Article34. Seiberg M, Paine C, Sharlow E, Andrade-Gordon P, Costanzo M, Eisinger M, et al. Inhibition of melanosome transfer results in skin lightening. J Invest Dermatol. 2000. 115:162–167.35. Hakozaki T, Minwalla L, Zhuang J, Chhoa M, Matsubara A, Miyamoto K, et al. The effect of niacinamide on reducing cutaneous pigmentation and suppression of melanosome transfer. Br J Dermatol. 2002. 147:20–31.
Article36. Imokawa G, Kobayashi T, Miyagishi M, Higashi K, Yada Y. The role of endothelin-1 in epidermal hyperpigmentation and signaling mechanisms of mitogenesis and melanogenesis. Pigment Cell Res. 1997. 10:218–228.
Article37. Kligman AM, Willis I. A new formula for depigmenting human skin. Arch Dermatol. 1975. 111:40–48.
Article38. Yokota T, Nishio H, Kubota Y, Mizoguchi M. The inhibitory effect of glabridin from licorice extracts on melanogenesis and inflammation. Pigment Cell Res. 1998. 11:355–361.
Article39. Karg E, Odh G, Wittbjer A, Rosengren E, Rorsman H. Hydrogen peroxide as an inducer of elevated tyrosinase level in melanoma cells. J Invest Dermatol. 1993. 100:2 Suppl. 209S–213S.
Article40. Gukasyan GS. Study of the kinetics of oxidation of monophenols by tyrosinase. The effect of reducers. Biochemistry (Mosc). 2002. 67:277–280.41. Ros JR, Rodríguez-López JN, García-Cánovas F. Effect of L-ascorbic acid on the monophenolase activity of tyrosinase. Biochem J. 1993. 295:309–312.
Article42. Ichihashi M, Funasaka Y, Ohashi A, Chacraborty A, Ahmed NU, Ueda M, et al. The inhibitory effect of DL-alpha-tocopheryl ferulate in lecithin on melanogenesis. Anticancer Res. 1999. 19:3769–3774.43. Nishiyama T, Ohnishi J, Hashiguchi Y. Fused heterocyclic antioxidants: antioxidative activities of hydrocoumarins in a homogeneous solution. Biosci Biotechnol Biochem. 2001. 65:1127–1133.
Article44. Yamamura T, Onishi J, Nishiyama T. Antimelanogenic activity of hydrocoumarins in cultured normal human melanocytes by stimulating intracellular glutathione synthesis. Arch Dermatol Res. 2002. 294:349–354.
Article45. Saliou C, Kitazawa M, McLaughlin L, Yang JP, Lodge JK, Tetsuka T, et al. Antioxidants modulate acute solar ultraviolet radiation-induced NF-kappa-B activation in a human keratinocyte cell line. Free Radic Biol Med. 1999. 26:174–183.
Article46. d'Ischia M, Napolitano A, Prota G. Peroxidase as an alternative to tyrosinase in the oxidative polymerization of 5,6-dihydroxyindoles to melanin(s). Biochim Biophys Acta. 1991. 1073:423–430.47. Kasraee B. Peroxidase-mediated mechanisms are involved in the melanocytotoxic and melanogenesis-inhibiting effects of chemical agents. Dermatology. 2002. 205:329–339.
Article48. Kasraee B. Depigmentation of brown Guinea pig skin by topical application of methimazole. J Invest Dermatol. 2002. 118:205–207.
Article49. Park SH, Kim DS, Kim WG, Ryoo IJ, Lee DH, Huh CH, et al. Terrein: a new melanogenesis inhibitor and its mechanism. Cell Mol Life Sci. 2004. 61:2878–2885.
Article50. Park SH, Kim DS, Lee HK, Kwon SB, Lee S, Ryoo IJ, et al. Long-term suppression of tyrosinase by terrein via tyrosinase degradation and its decreased expression. Exp Dermatol. 2009. 18:562–566.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Insights into the Molecular and Cellular Mechanism of Antipsychotics
- Comparative Study of Glycolic Acid Peeling vs. Tretinoin Peeling in Facial Pigmentary Lesions
- Topical Treatment of Onychomycosis
- Sodium-Glucose Cotransporter 2 Inhibitors: Mechanisms of Action and Various Effects
- Development History of Antipsychotic Drugs