Ann Dermatol.
2011 May;23(2):217-221. 10.5021/ad.2011.23.2.217.
Exaggeration of Wrinkles after Botulinum Toxin Injection for Forehead Horizontal Lines
- Affiliations
-
- 1Department of Dermatology, Asan Medical Center, Seoul, Korea. blue9854@medimail.co.kr
- 2Chicago College of Osteopathic Medicine, Midwestern University, Downers Grove, IL, USA.
- 3Yemiwon Aesthetic Clinic, Seoul, Korea.
- 4Department of Dermatology, Seoul National University College of Medicine, Seoul, Korea.
- 5Sense Dermatologic Clinic, Seoul, Korea.
- KMID: 2156667
- DOI: http://doi.org/10.5021/ad.2011.23.2.217
Abstract
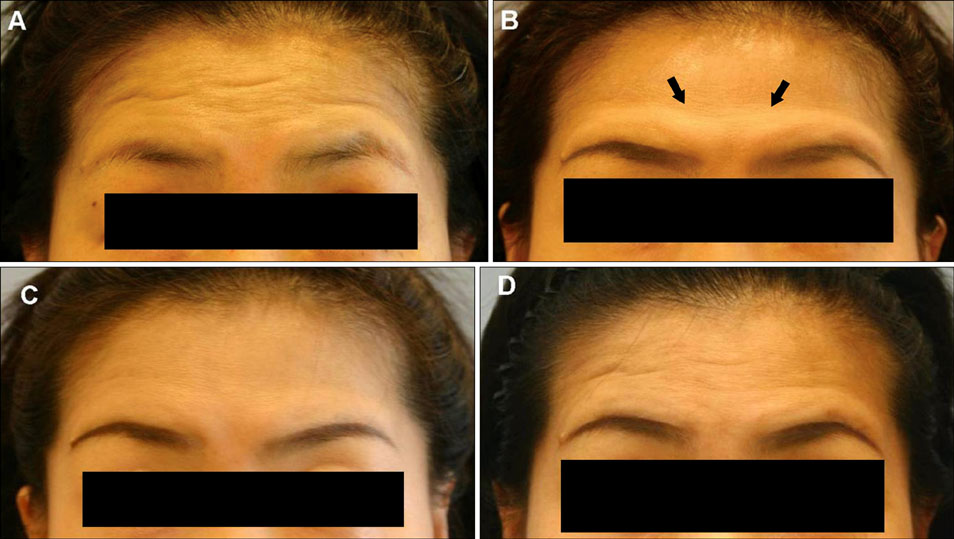
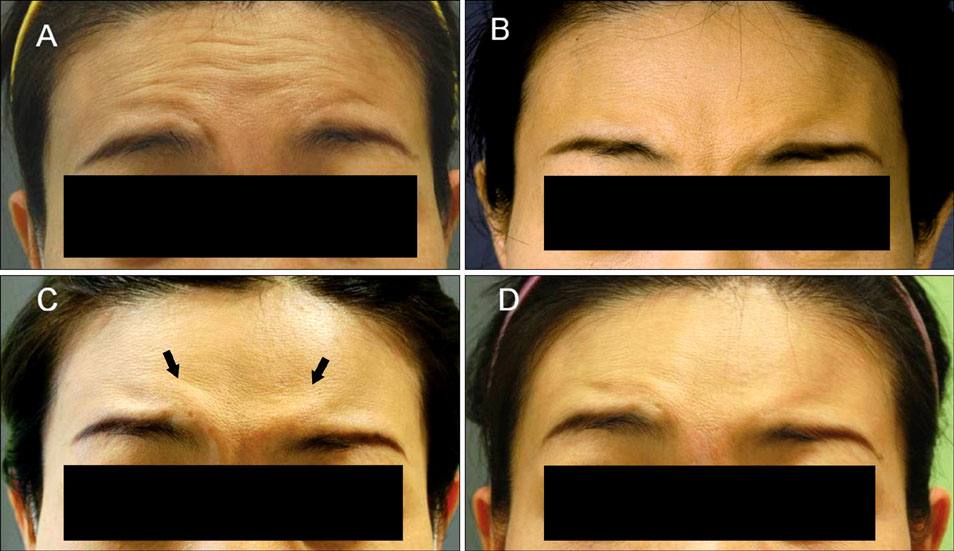
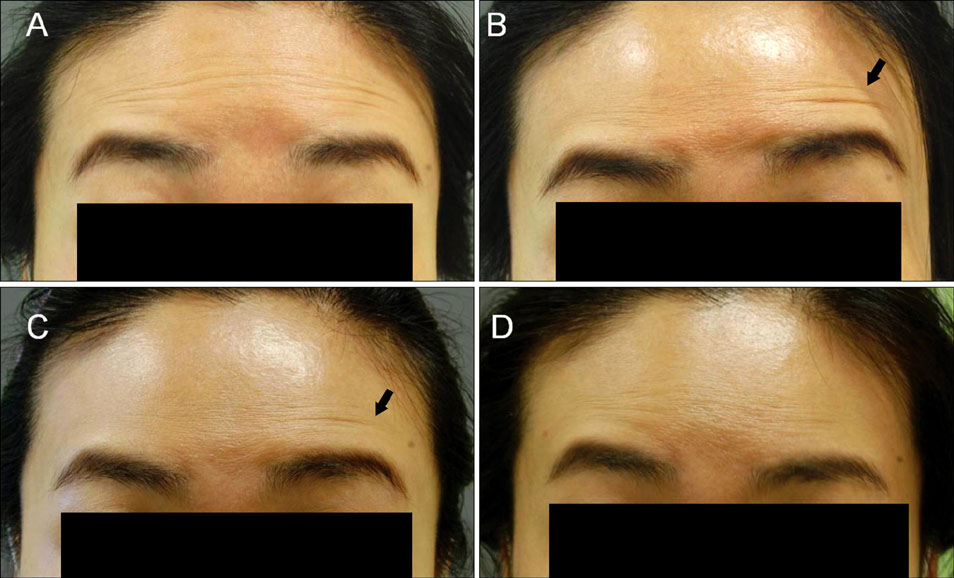
- There have been no long-term complications or life-threatening adverse effects related to botulinum toxin treatment for any cosmetic indications. Nevertheless, there are well-known, mild side effects of botulinum toxin treatment on the upper face, though most of them are self limited with time. However, excluding brow ptosis, reports about site specific side effects are few and anecdotal. We experienced cases of exaggeration of wrinkles after botulinum toxin injection for forehead horizontal lines, and report them here. In our cases, new appearance of a noticeable glabellar protrusion following botulinum toxin injection on the forehead was observed in 2 patients. Also, a new deep wrinkle on one side of the forehead just above the eyebrow appeared in another 2 patients. The exaggerated wrinkles nearly disappeared without treatment by week 4 in all subjects. These exaggerations of wrinkles may be caused by hyperactivity and overcompensation of untreated muscles. With the increasing availability of diverse botulinum toxin for cosmetic purposes, physicians and patients should be aware of this temporary change after therapeutic injections. We recommend explaining this possible effect prior to injection, for better understanding of treatment for cosmetic indications.
Figure
Reference
-
1. Carruthers A, Carruthers J, Cohen J. A prospective, double-blind, randomized, parallel- group, dose-ranging study of botulinum toxin type a in female subjects with horizontal forehead rhytides. Dermatol Surg. 2003. 29:461–467.
Article2. Carruthers JA, Lowe NJ, Menter MA, Gibson J, Nordquist M, Mordaunt J, et al. A multicenter, double-blind, randomized, placebo-controlled study of the efficacy and safety of botulinum toxin type A in the treatment of glabellar lines. J Am Acad Dermatol. 2002. 46:840–849.
Article3. Carruthers JA, Lowe NJ, Menter MA, Gibson J, Nordquist M, Mordaunt J, et al. One-year, randomized, multicenter, two-period study of the safety and efficacy of repeated treatments with botulinum toxin type A in patients with glabellar lines. J Clin Res. 2004. 7:1–20.4. Hambleton P, Moore AP. Moore P, editor. Botulinum neurotoxins: origin, structure, molecular actions and antibodies. Handbook of botulinum toxin treatment. 1995. 1st ed. Oxford: Blackwell Science;16–27.5. Rzany B, Dill-Müller D, Grablowitz D, Heckmann M, Caird D. Repeated botulinum toxin A injections for the treatment of lines in the upper face: a retrospective study of 4,103 treatments in 945 patients. Dermatol Surg. 2007. 33:S18–S25.
Article6. Youn SW, Seo KI, Yoo JY, Park KC, Eun HC. A clinical study of facial wrinkles affected by facial expression muscles treated with botulinum toxin (Botox®). Korean J Dermatol. 2002. 40:386–392.7. Ascher B, Talarico S, Cassuto D, Escobar S, Hexsel D, Jaén P, et al. International consensus recommendations on the aesthetic usage of botulinum toxin type A (Speywood Unit)--Part I: Upper facial wrinkles. J Eur Acad Dermatol Venereol. 2010. 24:1278–1284.
Article8. Klein AW. Complications, adverse reactions, and insights with the use of botulinum toxin. Dermatol Surg. 2003. 29:549–556.
Article9. Blitzer A, Binder WJ, Aviv JE, Keen MS, Brin MF. The management of hyperfunctional facial lines with botulinum toxin. A collaborative study of 210 injection sites in 162 patients. Arch Otolaryngol Head Neck Surg. 1997. 123:389–392.
Article10. Klein AW. Cosmetic therapy with botulinum toxin, anecdotal memoirs. Dermatol Surg. 1996. 22:757–759.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Botulinum Toxin Injection in Plastic Surgery
- Intradermal Injection of Botulinum Toxin: A Safer Treatment Modality for Forehead Wrinkles
- The Effects of Botulinum Toxin (BTXA(R)) Dermal Injections on Facial Wrinkle Lines
- Botulinum Toxin Type A for Facial Wrinkles and Benign Masseter Hypertrophy in Korean Patients
- Severe anaphylactic facial edema after botulinum toxin injections for frontal wrinkles: a case report