Korean J Crit Care Med.
2015 Nov;30(4):286-294. 10.4266/kjccm.2015.30.4.286.
Perioperative Risk Factors associated with Immediate Postoperative Extracorporeal Membrane Oxygenation in Lung Transplants
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. Anesjeongmin@yuhs.ac
- 2Department of Thoracic and Cardiovascular Surgery, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Anesthesiology and Pain Medicine, CHA Bundang Medical Center, CHA University, Seongnam, Korea.
- KMID: 2156182
- DOI: http://doi.org/10.4266/kjccm.2015.30.4.286
Abstract
- BACKGROUND
Extracorporeal membrane oxygenation (ECMO) is administered for a few days after lung transplantation (LTx) in recipients who are expected to have early graft dysfunction. Despite its life-saving potential, immediate postoperative ECMO has life-threatening complications such as postoperative bleeding. We investigated the risk factors related to the use of immediate postoperative ECMO.
METHODS
We retrospectively reviewed the records of 60 LTx patients who were at our institution from October 2012 to May 2015. Perioperative variables associated with postoperative ECMO were compared between the two groups.
RESULTS
There were 26 patients who received postoperative ECMO (ECMO group) and 34 patients who did not (control group). Multivariate regression analysis revealed preoperative ECMO (odds ratio [OR] 12.55, 95% confidence intervals [CI] 1.34 - 117.24, p = 0.027) and lower peripheral pulse oxymetry saturation (SpO2) at the end of surgery (OR 0.71, 95% CI 0.54 - 0.95, p = 0.019) were independent risk factors for postoperative ECMO in LTx patients. The incidences of complications, such as re-operation, tracheostomy, renal failure and postoperative atrial fibrillation, were higher in the ECMO group. There was no difference in the duration of postoperative intensive care unit stay or postoperative 30-day mortality between the two groups.
CONCLUSIONS
The preoperative ECMO and lower SpO2 at the end of surgery were associated with postoperative ECMO. Further, postoperative adverse events were higher in the ECMO group compared with the control group. This study suggests that determination of postoperative ECMO requires careful consideration because of the risks of postoperative ECMO in LTx patients.
Keyword
MeSH Terms
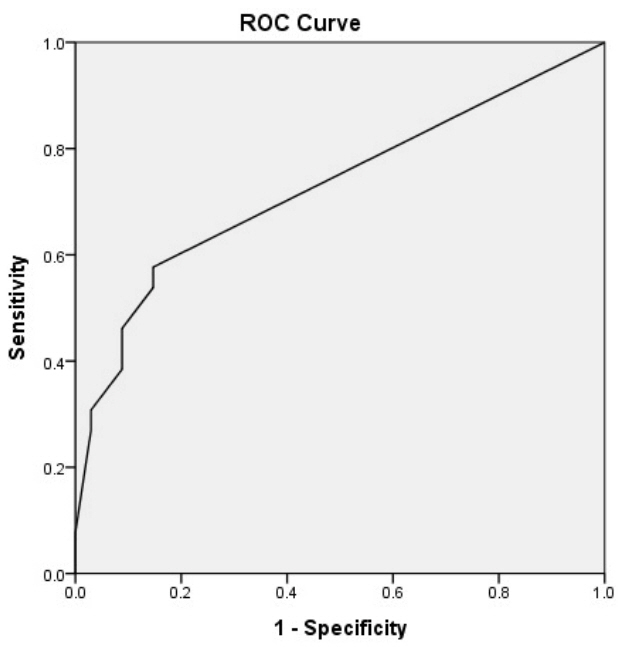
Figure
Cited by 1 articles
-
The Future of Research on Extracorporeal Membrane Oxygenation (ECMO)
Ji Young Lee
Korean J Crit Care Med. 2016;31(2):73-75. doi: 10.4266/kjccm.2016.31.2.73.
Reference
-
References
1. Hardy JD, Webb WR, Dalton ML Jr, Walker GR Jr. Lung homotransplantation in man. JAMA. 1963; 186:1065–74.
Article2. Yusen RD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant. 2014; 33:1009–24.
Article3. Ius F, Kuehn C, Tudorache I, Sommer W, Avsar M, Boethig D, et al. Lung transplantation on cardiopulmonary support: venoarterial extracorporeal membrane oxygenation outperformed cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2012; 144:1510–6.
Article4. Aigner C, Wisser W, Taghavi S, Lang G, Jaksch P, Czyzewski D, et al. Institutional experience with extracorporeal membrane oxygenation in lung transplantation. Eur J Cardiothorac Surg. 2007; 31:468–73.
Article5. Zangrillo A, Landoni G, Biondi-Zoccai G, Greco M, Greco T, Frati G, et al. A meta-analysis of complications and mortality of extracorporeal membrane oxygenation. Crit Care Resusc. 2013; 15:172–8.6. Lee YJ, Kim DJ, Kim JS, Lee JH, Lee CT, Jheon S, et al. Experience and results with VV-ECMO for severe acute respiratory failure: weaning versus nonweaning. ASAIO J. 2015; 61:184–9.7. Fischer S, Bohn D, Rycus P, Pierre AF, de Perrot M, Waddell TK, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: analysis of the Extracorporeal Life Support Organization (ELSO) registry. J Heart Lung Transplant. 2007; 26:472–7.
Article8. Gulack BC, Hirji SA, Hartwig MG. Bridge to lung transplantation and rescue post-transplant: the expanding role of extracorporeal membrane oxygenation. J Thorac Dis. 2014; 6:1070–9.9. Glassman LR, Keenan RJ, Fabrizio MC, Sonett JR, Bierman MI, Pham SM, et al. Extracorporeal membrane oxygenation as an adjunct treatment for primary graft failure in adult lung transplant recipients. J Thorac Cardiovasc Surg. 1995; 110:723–6.
Article10. Hartwig MG, Walczak R, Lin SS, Davis RD. Improved survival but marginal allograft function in patients treated with extracorporeal membrane oxygenation after lung transplantation. Ann Thorac Surg. 2012; 93:366–71.
Article11. Lentner C. Geigy Scientific Tables, Vol. 2: Introduction to Statistics Statistical Tables Mathematical Formulae. 8th ed. Basle, Switzerland: Ciba-Geigy;1982. p. 156–62. 163.12. Bermudez CA, Adusumilli PS, McCurry KR, Zaldonis D, Crespo MM, Pilewski JM, et al. Extracorporeal membrane oxygenation for primary graft dysfunction after lung transplantation: long-term survival. Ann Thorac Surg. 2009; 87:854–60.
Article13. Castleberry AW, Hartwig MG, Whitson BA. Extracorporeal membrane oxygenation post lung transplantation. Curr Opin Organ Transplant. 2013; 18:524–30.
Article14. Oto T, Rosenfeldt F, Rowland M, Pick A, Rabinov M, Preovolos A, et al. Extracorporeal membrane oxygenation after lung transplantation: evolving technique improves outcomes. Ann Thorac Surg. 2004; 78:1230–5.
Article15. Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D; ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005; 24:1454–9.
Article16. Nosotti M, Palleschi A, Rosso L, Tosi D, Mendogni P, Righi I, et al. Clinical risk factors for primary graft dysfunction in a low-volume lung transplantation center. Transplant Proc. 2014; 46:2329–33.
Article17. Toyoda Y, Bhama JK, Shigemura N, Zaldonis D, Pilewski J, Crespo M, et al. Efficacy of extracorporeal membrane oxygenation as a bridge to lung transplantation. J Thorac Cardiovasc Surg. 2013; 145:1065–70.
Article18. Meyers BF, Sundt TM, Henry S, Trulock EP, Guthrie T, Cooper JD, et al. Selective use of extracorporeal membrane oxygenation is warranted after lung transplantation. J Thorac Cardiovasc Surg. 2000; 120:20–6.
Article19. Hartwig MG, Appel JZ 3rd, Cantu E 3rd, Simsir S, Lin SS, Hsieh CC, et al. Improved results treating lung allograft failure with venovenous extracorporeal membrane oxygenation. Ann Thorac Surg. 2005; 80:1872–80.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sequential Bilateral Lung Transplantation with Extracorporeal Membrane Oxygenation (ECMO) Support: A case report
- Whole Lung Lavage in Pulmonary Alveolar Proteinosis associated with Lung Cancer Using Extracorporeal Membrane Oxygenation (ECMO)
- Whole Lung Lavage and Extracorporeal Membrane Oxygenation in a Patient with Pulmonary Alveolar Proteinosis and Lung Cancer
- Successful Retrieval of a Fractured Guidewire during Extracorporeal Membrane Oxygenator Insertion
- Extracorporeal Membrane Oxygenation Therapy for Aspiration Pneumonia in a Patient following Left Pneumonectomy for Lung Cancer