Korean J Radiol.
2015 Jun;16(3):617-625. 10.3348/kjr.2015.16.3.617.
Effect of Arterial Deprivation on Growing Femoral Epiphysis: Quantitative Magnetic Resonance Imaging Using a Piglet Model
- Affiliations
-
- 1Department of Radiology, Seoul National University College of Medicine, and Institute of Radiation Medicine, SNUMRC, Seoul 110-744, Korea.
- 2Department of Orthopedic Surgery, Seoul National University College of Medicine, Seoul 110-744, Korea. yoowj@snu.ac.kr
- KMID: 2155532
- DOI: http://doi.org/10.3348/kjr.2015.16.3.617
Abstract
OBJECTIVE
To investigate the usefulness of dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and diffusion MRI for the evaluation of femoral head ischemia.
MATERIALS AND METHODS
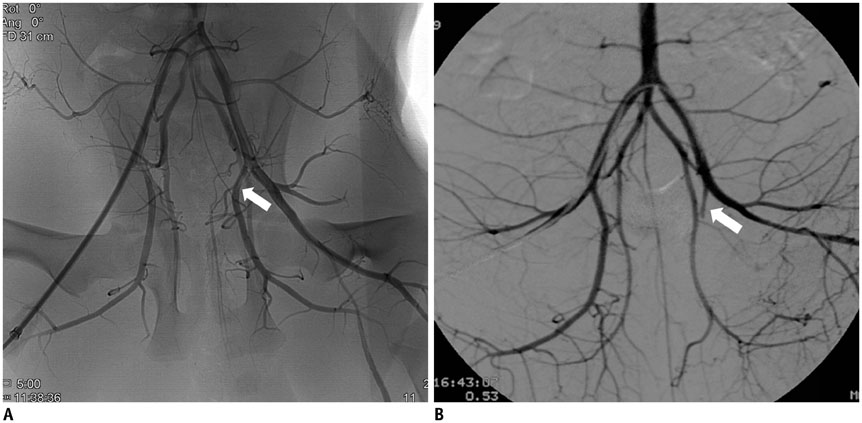
Unilateral femoral head ischemia was induced by selective embolization of the medial circumflex femoral artery in 10 piglets. All MRIs were performed immediately (1 hour) and after embolization (1, 2, and 4 weeks). Apparent diffusion coefficients (ADCs) were calculated for the femoral head. The estimated pharmacokinetic parameters (Kep and Ve from two-compartment model) and semi-quantitative parameters including peak enhancement, time-to-peak (TTP), and contrast washout were evaluated.
RESULTS
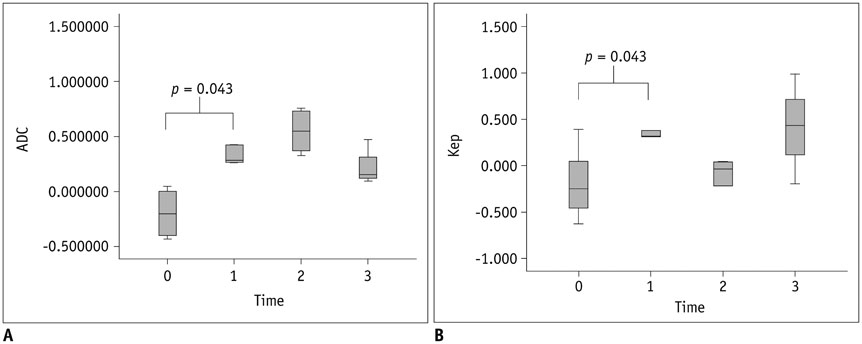
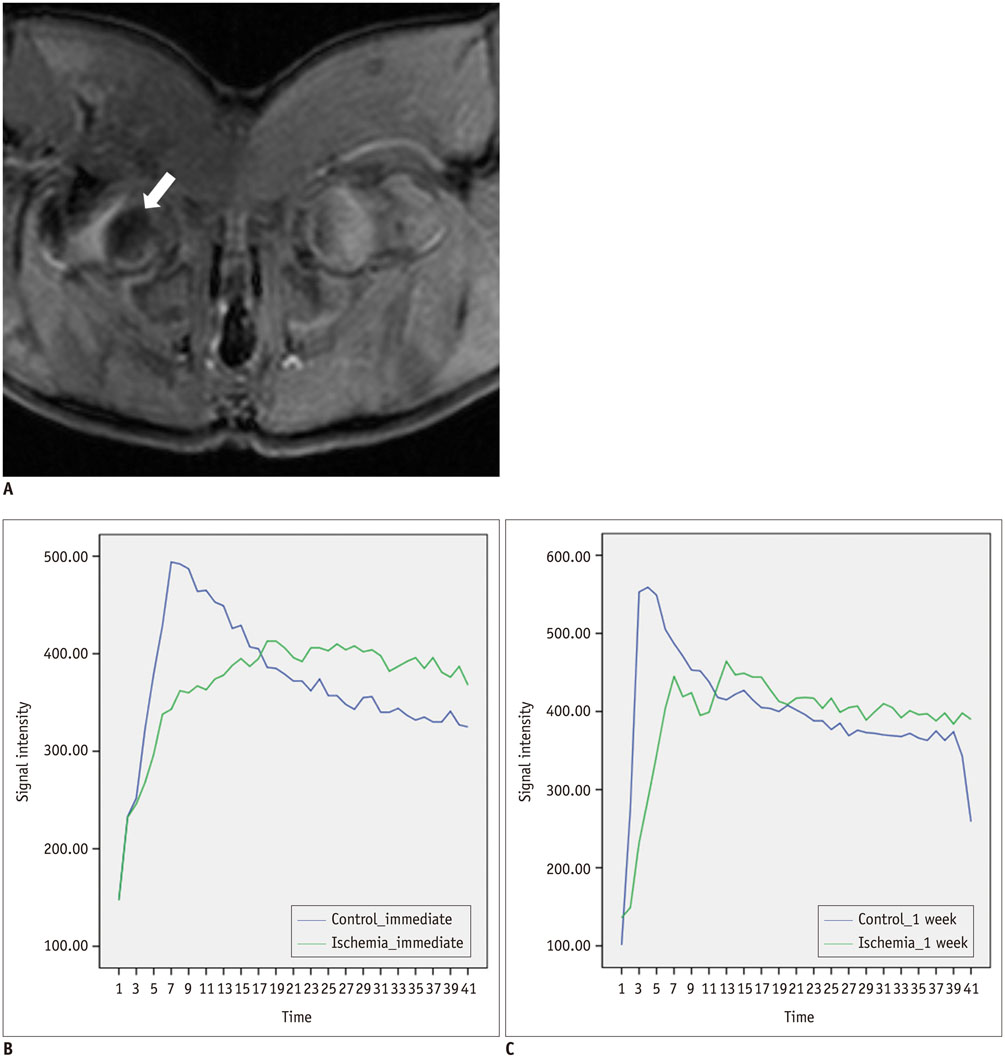
The epiphyseal ADC values of the ischemic hip decreased immediately (1 hour) after embolization. However, they increased rapidly at 1 week after embolization and remained elevated until 4 weeks after embolization. Perfusion MRI of ischemic hips showed decreased epiphyseal perfusion with decreased Kep immediately after embolization. Signal intensity-time curves showed delayed TTP with limited contrast washout immediately post-embolization. At 1-2 weeks after embolization, spontaneous reperfusion was observed in ischemic epiphyses. The change of ADC (p = 0.043) and Kep (p = 0.043) were significantly different between immediate (1 hour) after embolization and 1 week post-embolization.
CONCLUSION
Diffusion MRI and pharmacokinetic model obtained from the DCE-MRI are useful in depicting early changes of perfusion and tissue damage using the model of femoral head ischemia in skeletally immature piglets.
MeSH Terms
Figure
Reference
-
1. Kim HK, Herring JA. Pathophysiology, classifications, and natural history of Perthes disease. Orthop Clin North Am. 2011; 42:285–295. v2. Kim HK. Pathophysiology and new strategies for the treatment of Legg-Calvé-Perthes disease. J Bone Joint Surg Am. 2012; 94:659–669.3. Boss JH, Misselevich I. Osteonecrosis of the femoral head of laboratory animals: the lessons learned from a comparative study of osteonecrosis in man and experimental animals. Vet Pathol. 2003; 40:345–354.4. Alpaslan AM, Aksoy MC, Yazici M. Interruption of the blood supply of femoral head: an experimental study on the pathogenesis of Legg-Calve-Perthes Disease. Arch Orthop Trauma Surg. 2007; 127:485–491.5. Norman D, Reis D, Zinman C, Misselevich I, Boss JH. Vascular deprivation-induced necrosis of the femoral head of the rat. An experimental model of avascular osteonecrosis in the skeletally immature individual or Legg-Perthes disease. Int J Exp Pathol. 1998; 79:173–181.6. Kim HK, Stephenson N, Garces A, Aya-ay J, Bian H. Effects of disruption of epiphyseal vasculature on the proximal femoral growth plate. J Bone Joint Surg Am. 2009; 91:1149–1158.7. Li X, Qi J, Xia L, Yu C, Peng W, Hu X, et al. Dynamic gadolinium-enhanced MRI in early ischaemia of the proximal femoral epiphysis--a preliminary study. Clin Radiol. 2008; 63:1149–1159.8. Tsao AK, Dias LS, Conway JJ, Straka P. The prognostic value and significance of serial bone scintigraphy in Legg-Calvé-Perthes disease. J Pediatr Orthop. 1997; 17:230–239.9. Menezes NM, Connolly SA, Shapiro F, Olear EA, Jimenez RM, Zurakowski D, et al. Early ischemia in growing piglet skeleton: MR diffusion and perfusion imaging. Radiology. 2007; 242:129–136.10. Yoo WJ, Kim YJ, Menezes NM, Cheon JE, Jaramillo D. Diffusion-weighted MRI reveals epiphyseal and metaphyseal abnormalities in Legg-Calvé-Perthes disease: a pilot study. Clin Orthop Relat Res. 2011; 469:2881–2888.11. Sebag G, Ducou Le Pointe H, Klein I, Maiza D, Mazda K, Bensahel H, et al. Dynamic gadolinium-enhanced subtraction MR imaging--a simple technique for the early diagnosis of Legg-Calvé-Perthes disease: preliminary results. Pediatr Radiol. 1997; 27:216–220.12. Lamer S, Dorgeret S, Khairouni A, Mazda K, Brillet PY, Bacheville E, et al. Femoral head vascularisation in Legg-Calvé-Perthes disease: comparison of dynamic gadolinium-enhanced subtraction MRI with bone scintigraphy. Pediatr Radiol. 2002; 32:580–585.13. Courcoutsakis N, Spanoudaki A, Maris TG, Astrinakis E, Spanoudakis E, Tsatalas C, et al. Perfusion parameters analysis of the vertebral bone marrow in patients with Ph1- chronic myeloproliferative neoplasms (Ph(neg) MPN): a dynamic contrast-enhanced MRI (DCE-MRI) study. J Magn Reson Imaging. 2012; 35:696–702.14. De Coninck T, Jans L, Sys G, Huysse W, Verstraeten T, Forsyth R, et al. Dynamic contrast-enhanced MR imaging for differentiation between enchondroma and chondrosarcoma. Eur Radiol. 2013; 23:3140–3152.15. Lecouvet FE, Larbi A, Pasoglou V, Omoumi P, Tombal B, Michoux N, et al. MRI for response assessment in metastatic bone disease. Eur Radiol. 2013; 23:1986–1997.16. Boesen M, Kubassova O, Bouert R, Axelsen MB, Ostergaard M, Cimmino MA, et al. Correlation between computer-aided dynamic gadolinium-enhanced MRI assessment of inflammation and semi-quantitative synovitis and bone marrow oedema scores of the wrist in patients with rheumatoid arthritis--a cohort study. Rheumatology (Oxford). 2012; 51:134–143.17. Griffith JF, Yeung DK, Leung JC, Kwok TC, Leung PC. Prediction of bone loss in elderly female subjects by MR perfusion imaging and spectroscopy. Eur Radiol. 2011; 21:1160–1169.18. Budzik JF, Lefebvre G, Forzy G, El Rafei M, Chechin D, Cotten A. Study of proximal femoral bone perfusion with 3D T1 dynamic contrast-enhanced MRI: a feasibility study. Eur Radiol. 2014; 24:3217–3223.19. Chan WP, Liu YJ, Huang GS, Lin MF, Huang S, Chang YC, et al. Relationship of idiopathic osteonecrosis of the femoral head to perfusion changes in the proximal femur by dynamic contrast-enhanced MRI. AJR Am J Roentgenol. 2011; 196:637–643.20. Sabo E, Peskin B, Misselevich I, Zinman C, Levin D, Norman D, et al. Computer-assisted image analysis of the rat postosteonecrotic remodeled femoral head. Exp Mol Pathol. 2001; 71:256–264.21. Ferré JC, Shiroishi MS, Law M. Advanced techniques using contrast media in neuroimaging. Magn Reson Imaging Clin N Am. 2012; 20:699–713.22. Choi HS, Kim AH, Ahn SS, Shin NY, Kim J, Lee SK. Glioma grading capability: comparisons among parameters from dynamic contrast-enhanced MRI and ADC value on DWI. Korean J Radiol. 2013; 14:487–492.23. Law M, Young R, Babb J, Rad M, Sasaki T, Zagzag D, et al. Comparing perfusion metrics obtained from a single compartment versus pharmacokinetic modeling methods using dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol. 2006; 27:1975–1982.24. Brix G, Semmler W, Port R, Schad LR, Layer G, Lorenz WJ. Pharmacokinetic parameters in CNS Gd-DTPA enhanced MR imaging. J Comput Assist Tomogr. 1991; 15:621–628.25. Tofts PS, Kermode AG. Measurement of the blood-brain barrier permeability and leakage space using dynamic MR imaging. 1. Fundamental concepts. Magn Reson Med. 1991; 17:357–367.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Responsible Factors for Femoral Shortening in Piglet Legg-Calve-Perthes Disease Models
- Pathologic Separation of Capital Femoral Epiphysis due to an Osteosarcoma
- Effect of Curettage and DBM-CaSO4 Graft for the Treatment of Ischemic Necrosis of the Capital Femoral Epiphysis in Immature Pigs
- A Clinical Study of Slipped Capital Femoral epiphysis
- Effects of Multiple Drilling on the Ischemic Capital Femoral Epiphysis of Immature Piglets