Ann Rehabil Med.
2016 Feb;40(1):81-87. 10.5535/arm.2016.40.1.81.
Usefulness of Transcranial Magnetic Stimulation to Assess Motor Function in Patients With Parkinsonism
- Affiliations
-
- 1Department of Physical and Rehabilitation Medicine, Center for Prevention and Rehabilitation, Heart Vascular and Stroke Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. wh.chang@samsung.com
- 2Department of Neurology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Health Science and Technology, SAIHST, Sungkyunkwan University, Seoul, Korea.
- 4Department of Medical Device Management & Research, SAIHST, Sungkyunkwan University, Seoul, Korea.
- KMID: 2155169
- DOI: http://doi.org/10.5535/arm.2016.40.1.81
Abstract
OBJECTIVE
To investigate the clinical significance of upper and lower extremity transcranial magnetic stimulation (TMS)-induced motor evoked potentials (MEPs) in patients with parkinsonism.
METHODS
Twenty patients (14 men, 6 women; mean age 70.5±9.1 years) suffering from parkinsonism were included in this study. All participants underwent single-pulse TMS session to assess the corticospinal excitability of the upper and lower extremity motor cortex. The resting motor threshold (RMT) was defined as the lowest stimulus intensity able to evoke MEPs of an at least 50 µV peak-to-peak amplitude in 5 of 10 consecutive trials. Five sweeps of MEPs at 120% of the RMT were performed, and the mean amplitude and latency of the MEPs were calculated. Patients were also assessed using the Unified Parkinson's Disease Rating Scale part III (UPDRS-III) and the 5-meter Timed Up and Go (5m-TUG) test.
RESULTS
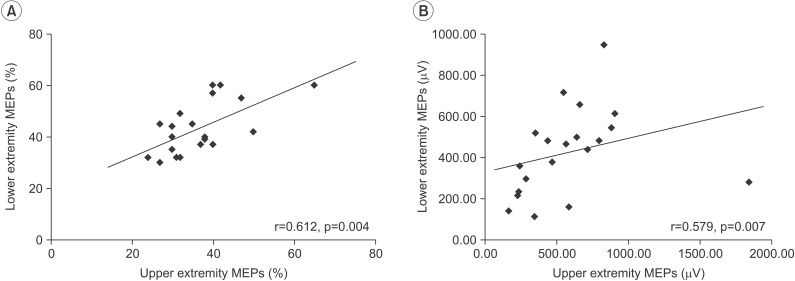
There was a significant positive correlation between the RMTs of MEPs in the upper and lower extremities (r=0.612, p=0.004) and between the amplitude of MEPs in the upper and lower extremities (r=0.579, p=0.007). The RMT of upper extremity MEPs showed a significant negative relationship with the UPDRS-III score (r=-0.516, p=0.020). In addition, RMTs of lower extremity MEPs exhibited a negative relationship with the UPDRS-III score, but the association was not statistically significant (r=-406, p=0.075).
CONCLUSION
These results indicated that the RMT of MEPs reflect the severity of motor dysfunction in patients with parkinsonism. MEP is a potential quantitative, electrodiagnostic method to assess motor function in patients with parkinsonism.
Keyword
MeSH Terms
Figure
Reference
-
1. Bestmann S, Krakauer JW. The uses and interpretations of the motor-evoked potential for understanding behaviour. Exp Brain Res. 2015; 233:679–689. PMID: 25563496.
Article2. Pennisi G, Ferri R, Cantone M, Lanza G, Pennisi M, Vinciguerra L, et al. A review of transcranial magnetic stimulation in vascular dementia. Dement Geriatr Cogn Disord. 2011; 31:71–80. PMID: 21242688.
Article3. List J, Kubke JC, Lindenberg R, Kulzow N, Kerti L, Witte V, et al. Relationship between excitability, plasticity and thickness of the motor cortex in older adults. Neuroimage. 2013; 83:809–816. PMID: 23876242.
Article4. Di Lazzaro V, Oliviero A, Profice P, Ferrara L, Saturno E, Pilato F, et al. The diagnostic value of motor evoked potentials. Clin Neurophysiol. 1999; 110:1297–1307. PMID: 10423196.
Article5. Curra A, Modugno N, Inghilleri M, Manfredi M, Hallett M, Berardelli A. Transcranial magnetic stimulation techniques in clinical investigation. Neurology. 2002; 59:1851–1859. PMID: 12503582.
Article6. Groppa S, Oliviero A, Eisen A, Quartarone A, Cohen LG, Mall V, et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clin Neurophysiol. 2012; 123:858–882. PMID: 22349304.
Article7. Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003; 2:145–156. PMID: 12849236.
Article8. Wirdefeldt K, Adami HO, Cole P, Trichopoulos D, Mandel J. Epidemiology and etiology of Parkinson's disease: a review of the evidence. Eur J Epidemiol. 2011; 26(Suppl 1):S1–S58. PMID: 21626386.
Article9. Dickson DW. Parkinson's disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med. 2012; 2:a009258. PMID: 22908195.
Article10. Kluger BM, Brown RP, Aerts S, Schenkman M. Determinants of objectively measured physical functional performance in early to mid-stage Parkinson disease. PM R. 2014; 6:992–998. PMID: 24880056.
Article11. Salawu FK, Danburam A, Olokoba AB. Non-motor symptoms of Parkinson's disease: diagnosis and management. Niger J Med. 2010; 19:126–131. PMID: 20642073.
Article12. Stebbins GT, Goetz CG. Factor structure of the Unified Parkinson's Disease Rating Scale: Motor Examination section. Mov Disord. 1998; 13:633–636. PMID: 9686766.
Article13. Lee SY, Kim MS, Chang WH, Cho JW, Youn JY, Kim YH. Effects of repetitive transcranial magnetic stimulation on freezing of gait in patients with Parkinsonism. Restor Neurol Neurosci. 2014; 32:743–753. PMID: 25079979.
Article14. Kim YH, You SH, Ko MH, Park JW, Lee KH, Jang SH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006; 37:1471–1476. PMID: 16675743.
Article15. Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): status and recommendations. Mov Disord. 2003; 18:738–750. PMID: 12815652.16. Bryant MS, Rintala DH, Graham JE, Hou JG, Protas EJ. Determinants of use of a walking device in persons with Parkinson's disease. Arch Phys Med Rehabil. 2014; 95:1940–1945. PMID: 24953250.
Article17. Goetz CG, Poewe W, Rascol O, Sampaio C, Stebbins GT, Counsell C, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004; 19:1020–1028. PMID: 15372591.18. Nascimbeni A, Gaffuri A, Granella L, Colli M, Imazio P. Prognostic value of motor evoked potentials in stroke motor outcome. Eura Medicophys. 2005; 41:125–130. PMID: 16200027.19. Ziemann U, Reis J, Schwenkreis P, Rosanova M, Strafella A, Badawy R, et al. TMS and drugs revisited 2014. Clin Neurophysiol. 2015; 126:1847–1868. PMID: 25534482.
Article20. Appel-Cresswell S, de la Fuente-Fernandez R, Galley S, McKeown MJ. Imaging of compensatory mechanisms in Parkinson's disease. Curr Opin Neurol. 2010; 23:407–412. PMID: 20610991.
Article21. Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, et al. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain. 2000; 123(Pt 2):394–403. PMID: 10648446.22. Kojovic M, Kassavetis P, Bologna M, Parees I, Rubio-Agusti I, Beraredelli A, et al. Transcranial magnetic stimulation follow-up study in early Parkinson's disease: a decline in compensation with disease progression? Mov Disord. 2015; 30:1098–1106. PMID: 25753906.
Article23. Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994; 91:79–92. PMID: 7519144.
Article24. Tremblay F, Tremblay LE. Cortico-motor excitability of the lower limb motor representation: a comparative study in Parkinson's disease and healthy controls. Clin Neurophysiol. 2002; 113:2006–2012. PMID: 12464341.
Article25. Bares M, Kanovsky P, Klajblova H, Rektor I. Intracortical inhibition and facilitation are impaired in patients with early Parkinson's disease: a paired TMS study. Eur J Neurol. 2003; 10:385–389. PMID: 12823490.26. Teo WP, Rodrigues JP, Mastaglia FL, Thickbroom GW. Modulation of corticomotor excitability after maximal or sustainable-rate repetitive finger movement is impaired in Parkinson's disease and is reversed by levodopa. Clin Neurophysiol. 2014; 125:562–568. PMID: 24095151.
Article27. Suppa A, Iezzi E, Conte A, Belvisi D, Marsili L, Modugno N, et al. Dopamine influences primary motor cortex plasticity and dorsal premotor-to-motor connectivity in Parkinson's disease. Cereb Cortex. 2010; 20:2224–2233. PMID: 20051362.
Article28. MacKinnon CD, Gilley EA, Weis-McNulty A, Simuni T. Pathways mediating abnormal intracortical inhibition in Parkinson's disease. Ann Neurol. 2005; 58:516–524. PMID: 16178015.
Article29. Chen R, Tam A, Butefisch C, Corwell B, Ziemann U, Rothwell JC, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. J Neurophysiol. 1998; 80:2870–2881. PMID: 9862891.
Article30. Brouwer B, Ashby P. Corticospinal projections to upper and lower limb spinal motoneurons in man. Electroencephalogr Clin Neurophysiol. 1990; 76:509–519. PMID: 1701119.
Article31. Luft AR, Smith GV, Forrester L, Whitall J, Macko RF, Hauser TK, et al. Comparing brain activation associated with isolated upper and lower limb movement across corresponding joints. Hum Brain Mapp. 2002; 17:131–140. PMID: 12353246.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Motor Evoked Potentials in Masseter, and Anterior Belly of Digastric Induced by Transcranial Magnetic Stimulation
- Clinical Usefulness of Silent Period after Transcranial Magnetic Stimulation in Stroke Patients
- Stroke Update 2011: Stroke Rehabilitation
- Motor Evoked Potentials by Transcranial Magnetic Stimulation
- Application of Non-invasive Brain Stimulation for Neurorehabilitation: Cerebellar Stimulation