Cancer Res Treat.
2016 Jan;48(1):288-296. 10.4143/crt.2014.297.
A Study of Relationship of Atheroembolic Risk Factors with Postoperative Recovery in Renal Function after Partial Nephrectomy in Patients Staged T1-2 Renal Cell Carcinoma during Median 4-Year Follow-up
- Affiliations
-
- 1Department of Urology, National Cancer Center, Goyang, Korea.
- 2Biometric Research Branch, National Cancer Center, Goyang, Korea.
- 3Department of Urology, Seoul National University Hospital, Seoul, Korea. eslee@snu.ac.kr
- 4Department of Cancer Control and Policy, Graduate School of Cancer Science and Policy, National Cancer Center, Goyang, Korea.
- KMID: 2152287
- DOI: http://doi.org/10.4143/crt.2014.297
Abstract
- PURPOSE
The objective of this study is to evaluate the relationship of atheroembolic risk factors with postoperative recovery of renal function after on-clamp partial nephrectomy (PN) with warm ischemia in patients with staged T1-2 renal cell carcinoma (RCC).
MATERIALS AND METHODS
A total of 234 patients from 2004 to 2012 were included, and their clinicopathologic and operative parameters, including atheroembolic risk factors were reviewed retrospectively. Renal function, as determined by estimated glomerular filtration rate (eGFR) and measurement of serum creatinine level (Cr) at each scheduled follow-up for a median four years, was compared between the high-risk (HR) group (n=49, > or = five risk factors) and the low-risk (LR) group (n=185, < five risk factors).
RESULTS
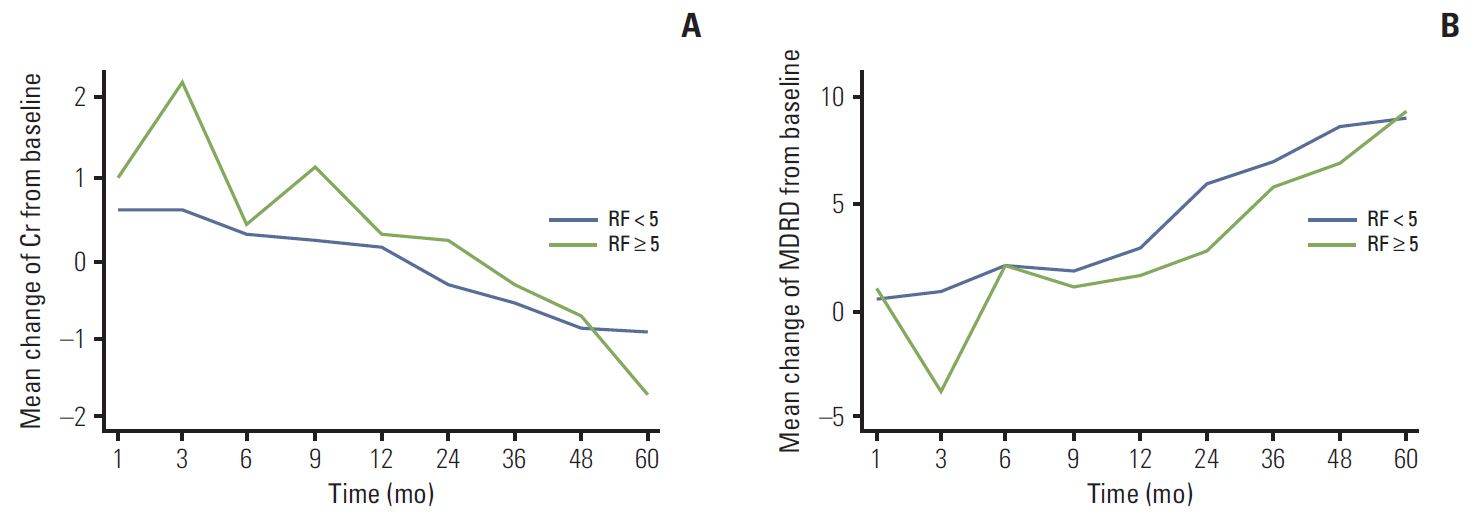
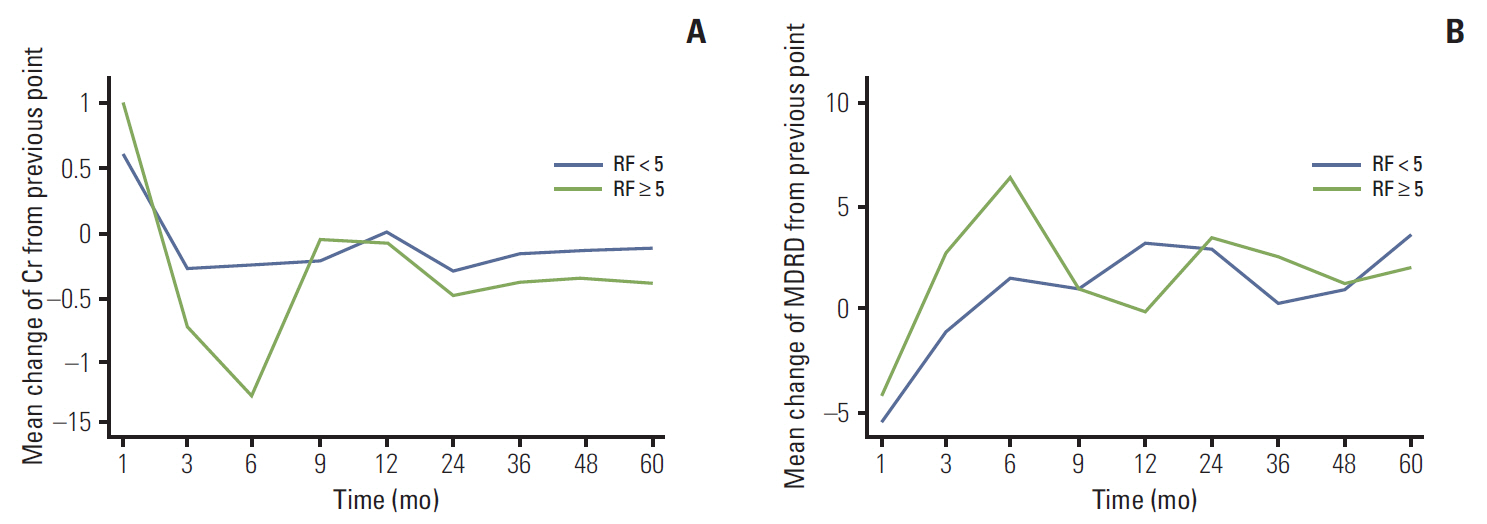
Except for baseline renal function and number of risk factors for atheroembolism, differences in characteristics between groups were comparatively insignificant. At 3 months after the operation, Cr and eGFR differed significantly between the two groups (p < 0.05), but no differences were observed afterward. Significant deterioration from baseline in Cr and eGFR was observed in both groups at 1 month after the operation, with a greater change in the HR group (p < 0.05). From measurement to measurement, significantly faster deterioration in Cr and eGFR was observed in the HR group than in the LR group until 6 months after the operation (Cr: LR, 0.02 mg/dL and HR, 0.13 mg/dL; eGFR: LR, 1.50 mL/min/1.73 m2 and HR, 6.38 mL/min/1.73 m2; p < 0.05).
CONCLUSION
The presence of atheroembolic risk factors may negatively influence postoperative recovery of renal function after PN in patients with localized RCC.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010; 58:398–406.
Article2. Leslie S, Goh AC, Gill IS. Partial nephrectomy: contemporary indications, techniques and outcomes. Nat Rev Urol. 2013; 10:275–83.3. Malcolm JB, Bagrodia A, Derweesh IH, Mehrazin R, Diblasio CJ, Wake RW, et al. Comparison of rates and risk factors for developing chronic renal insufficiency, proteinuria and metabolic acidosis after radical or partial nephrectomy. BJU Int. 2009; 104:476–81.
Article4. MacLennan S, Imamura M, Lapitan MC, Omar MI, Lam TB, Hilvano-Cabungcal AM, et al. Systematic review of perioperative and quality-of-life outcomes following surgical management of localised renal cancer. Eur Urol. 2012; 62:1097–117.
Article5. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Comparison of warm ischemia versus no ischemia during partial nephrectomy on a solitary kidney. Eur Urol. 2010; 58:331–6.
Article6. Kim SP, Thompson RH. Kidney function after partial nephrectomy: current thinking. Curr Opin Urol. 2013; 23:105–11.7. Kim SH, Lee SE, Hong SK, Jeong CW, Park YH, Kim YJ, et al. Incidence and risk factors of chronic kidney disease in korean patients with t1a renal cell carcinoma before and after radical or partial nephrectomy. Jpn J Clin Oncol. 2013; 43:1243–8.
Article8. Kates M, Badalato GM, McKiernan JM. Renal functional outcomes after surgery for renal cortical tumors. Curr Opin Urol. 2011; 21:351–5.
Article9. Thurlbeck WM, Castleman B. Atheromatous emboli to the kidneys after aortic surgery. N Engl J Med. 1957; 257:442–7.
Article10. Scolari F, Ravani P. Atheroembolic renal disease. Lancet. 2010; 375:1650–60.
Article11. Mayo RR, Swartz RD. Redefining the incidence of clinically detectable atheroembolism. Am J Med. 1996; 100:524–9.
Article12. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–70.13. Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009; 182:844–53.
Article14. de Mast Q, Beutler JJ. The prevalence of atherosclerotic renal artery stenosis in risk groups: a systematic literature review. J Hypertens. 2009; 27:1333–40.
Article15. Dumaine RL, Montalescot G, Steg PG, Ohman EM, Eagle K, Bhatt DL, et al. Renal function, atherothrombosis extent, and outcomes in high-risk patients. Am Heart J. 2009; 158:141–8.e1.
Article16. Meyrier A. Cholesterol crystal embolism: diagnosis and treatment. Kidney Int. 2006; 69:1308–12.
Article17. Scolari F, Ravani P, Pola A, Guerini S, Zubani R, Movilli E, et al. Predictors of renal and patient outcomes in atheroembolic renal disease: a prospective study. J Am Soc Nephrol. 2003; 14:1584–90.
Article18. Mittal BV, Alexander MP, Rennke HG, Singh AK. Atheroembolic renal disease: a silent masquerader. Kidney Int. 2008; 73:126–30.
Article19. Modi KS, Rao VK. Atheroembolic renal disease. J Am Soc Nephrol. 2001; 12:1781–7.
Article20. Frock J, Bierman M, Hammeke M, Reyes A. Atheroembolic renal disease: experience with 22 patients. Nebr Med J. 1994; 79:317–21.21. Hamscho N, Wilhelm A, Dobert N, Menzel C, Gossmann J, Berner U, et al. Residual kidney function after donor nephrectomy: assessment by 99mTc-MAG3-clearance. Nuklearmedizin. 2005; 44:200–4.22. Nizet A. The mechanisms of fast renal compensation. Pflugers Arch. 1973; 341:209–17.
Article23. Choi KH, Yoon YE, Kim KH, Han WK. Contralateral kidney volume change as a consequence of ipsilateral parenchymal atrophy promotes overall renal function recovery after partial nephrectomy. Int Urol Nephrol. 2015; 47:25–32.
Article24. Sharma N, O'Hara J, Novick AC, Lieber M, Remer EM, Herts BR. Correlation between loss of renal function and loss of renal volume after partial nephrectomy for tumor in a solitary kidney. J Urol. 2008; 179:1284–8.
Article25. Thompson RH, Lane BR, Lohse CM, Leibovich BC, Fergany A, Frank I, et al. Renal function after partial nephrectomy: effect of warm ischemia relative to quantity and quality of preserved kidney. Urology. 2012; 79:356–60.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Treatment Outcomes of a Partial Nephrectomy in the Management of Renal Cell Carcinomas
- Renal Function after Partial Nephrectomy for Renal Cell Carcinoma in Solitary Kidney
- Bilateral Synchronous Multifocal Renal Cell Carcinoma
- Non-ischemic Partial Nephrectomy with Using the Microwave Tissue Coagulator
- Percutaneous Embolization of Renal Artery Pseudoaneurysm after Laparoscopic Partial Nephrectomy for Renal Cell Carcinoma