J Cardiovasc Ultrasound.
2015 Dec;23(4):211-218. 10.4250/jcu.2015.23.4.211.
Predicting Peri-Device Leakage of Left Atrial Appendage Device Closure Using Novel Three-Dimensional Geometric CT Analysis
- Affiliations
-
- 1Division of Cardiology, Severance Cardiovascular Hospital, Yonsei University College of Medicine, Seoul, Korea. hjchang@yuhs.ac
- 2Yonsei-Cedar Sinai Integrative Cardiovascular Imaging Research Center, Seoul, Korea.
- 3Severance Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2144451
- DOI: http://doi.org/10.4250/jcu.2015.23.4.211
Abstract
- BACKGROUND
After left atrial appendage (LAA) device closure, peri-device leakage into the LAA persists due to incomplete occlusion. We hypothesized that pre-procedural three-dimensional (3D) geometric analysis of the interatrial septum (IAS) and LAA orifice can predict this leakage. We investigated the predictive parameters of LAA device closure obtained from baseline cardiac computerized tomography (CT) using a novel 3D analysis system.
METHODS
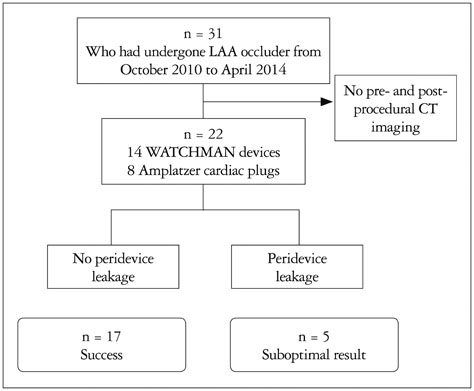
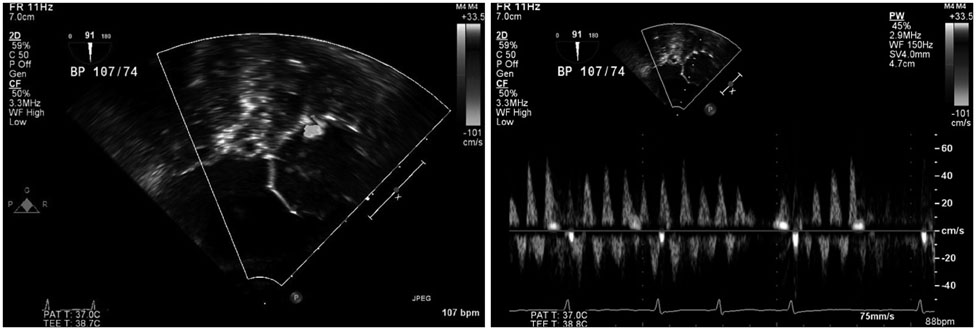
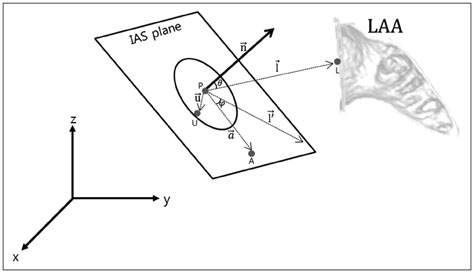
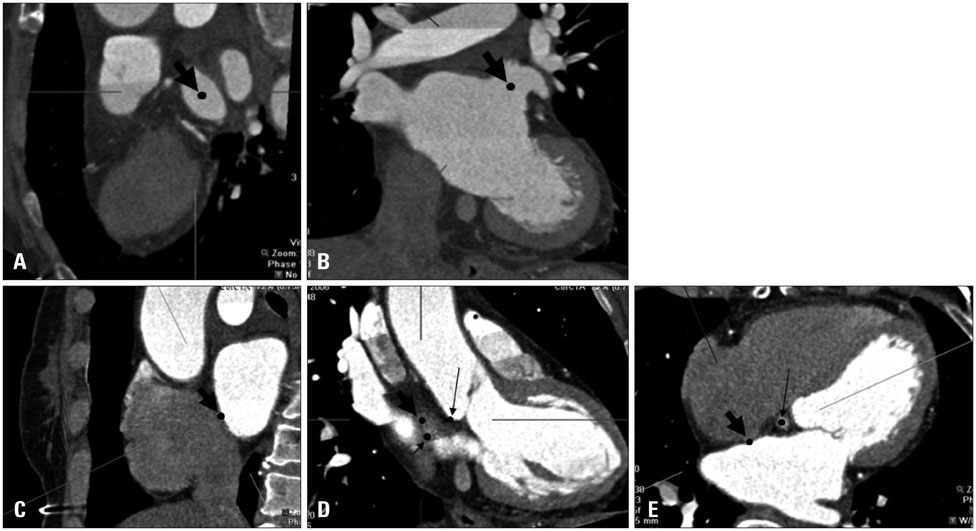
We conducted a retrospective study of 22 patients who underwent LAA device closure. We defined peri-device leakage as the presence of a Doppler signal inside the LAA after device deployment (group 2, n = 5) compared with patients without peri-device leakage (group 1, n = 17). Conventional parameters were measured by cardiac CT. Angles theta and phi were defined between the IAS plane and the line, linking the LAA orifice center and foramen ovale.
RESULTS
Group 2 exhibited significantly better left atrial (LA) function than group 1 (p = 0.031). Pre-procedural theta was also larger in this group (41.9degrees vs. 52.3degrees, p = 0.019). The LAA cauliflower-type morphology was more common in group 2. Overall, the patients' LA reserve significantly decreased after the procedure (21.7 mm3 vs. 17.8 mm3, p = 0.035). However, we observed no significant interval changes in pre- and post-procedural values of theta and phi in either group (all p > 0.05).
CONCLUSION
Angles between the IAS and LAA orifice might be a novel anatomical parameter for predicting peri-device leakage after LAA device closure. In addition, 3D CT analysis of the LA and LAA orifice could be used to identify clinically favorable candidates for LAA device closure.
Figure
Reference
-
1. Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, Fang MC, Fisher M, Furie KL, Heck DV, Johnston SC, Kasner SE, Kittner SJ, Mitchell PH, Rich MW, Richardson D, Schwamm LH, Wilson JA. American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014; 45:2160–2236.2. Reddy VY, Doshi SK, Sievert H, Buchbinder M, Neuzil P, Huber K, Halperin JL, Holmes D. PROTECT AF Investigators. Percutaneous left atrial appendage closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Circulation. 2013; 127:720–729.3. Urena M, Rodés-Cabau J, Freixa X, Saw J, Webb JG, Freeman M, Horlick E, Osten M, Chan A, Marquis JF, Champagne J, Ibrahim R. Percutaneous left atrial appendage closure with the AMPLATZER cardiac plug device in patients with nonvalvular atrial fibrillation and contraindications to anticoagulation therapy. J Am Coll Cardiol. 2013; 62:96–102.4. Viles-Gonzalez JF, Kar S, Douglas P, Dukkipati S, Feldman T, Horton R, Holmes D, Reddy VY. The clinical impact of incomplete left atrial appendage closure with the Watchman device in patients with atrial fibrillation: a PROTECT AF (percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation) substudy. J Am Coll Cardiol. 2012; 59:923–929.5. Fender EA, Sibley CT, Nazarian S, Cheng A, Spragg DD, Marine JE, Berger RD, Calkins H, Lima JA, Brinker JA, Henrikson CA. Atrial septal angulation varies widely in patients undergoing pulmonary vein isolation. J Invasive Cardiol. 2014; 26:128–131.6. Gage BF, van Walraven C, Pearce L, Hart RG, Koudstaal PJ, Boode BS, Petersen P. Selecting patients with atrial fibrillation for anticoagulation: stroke risk stratification in patients taking aspirin. Circulation. 2004; 110:2287–2292.7. Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010; 41:2731–2738.8. Veinot JP, Harrity PJ, Gentile F, Khandheria BK, Bailey KR, Eickholt JT, Seward JB, Tajik AJ, Edwards WD. Anatomy of the normal left atrial appendage: a quantitative study of age-related changes in 500 autopsy hearts: implications for echocardiographic examination. Circulation. 1997; 96:3112–3115.9. Aryana A, Saad EB, d'Avila A. Left atrial appendage occlusion and ligation devices: what is available, how to implement them, and how to manage and avoid complications. Curr Treat Options Cardiovasc Med. 2012; 14:503–519.10. De Backer O, Arnous S, Ihlemann N, Vejlstrup N, Jørgensen E, Pehrson S, Krieger TD, Meier P, Søndergaard L, Franzen OW. Percutaneous left atrial appendage occlusion for stroke prevention in atrial fibrillation: an update. Open Heart. 2014; 1:e000020.11. Wang Y, Di Biase L, Horton RP, Nguyen T, Morhanty P, Natale A. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. J Cardiovasc Electrophysiol. 2010; 21:973–982.12. Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Gallinghouse GJ, Burkhardt JD, Cesarani F, Scaglione M, Natale A, Gaita F. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. J Am Coll Cardiol. 2012; 60:531–538.13. Ho IC, Neuzil P, Mraz T, Beldova Z, Gross D, Formanek P, Taborsky M, Niederle P, Ruskin JN, Reddy VY. Use of intracardiac echocardiography to guide implantation of a left atrial appendage occlusion device (PLAATO). Heart Rhythm. 2007; 4:567–571.14. Mráz T, Neuzil P, Mandysová E, Niederle P, Reddy VY. Role of echocardiography in percutaneous occlusion of the left atrial appendage. Echocardiography. 2007; 24:401–404.15. Blendea D, Heist EK, Danik SB, Barrett C, Ruskin JN, Mansour M. Analysis of the left atrial appendage morphology by intracardiac echocardiography in patients with atrial fibrillation. J Interv Card Electrophysiol. 2011; 31:191–196.16. Bai R, Horton RP, DI Biase L, Mohanty P, Pump A, Cardinal D, Scallon C, Mohanty S, Santangeli P, Brantes MC, Sanchez J, Burkhardt JD, Zagrodzky JD, Gallinghouse GJ, Natale A. Intraprocedural and long-term incomplete occlusion of the left atrial appendage following placement of the WATCHMAN device: a single center experience. J Cardiovasc Electrophysiol. 2012; 23:455–461.17. Merchant FM, Delurgio DB. Site-specific transseptal cardiac catheterization guided by intracardiac echocardiography for emerging electrophysiology applications. J Innov Cardiac Rhythm Manage. 2013; 4:1415–1427.18. Bayard YL, Omran H, Neuzil P, Thuesen L, Pichler M, Rowland E, Ramondo A, Ruzyllo W, Budts W, Montalescot G, Brugada P, Serruys PW, Vahanian A, Piéchaud JF, Bartorelli A, Marco J, Probst P, Kuck KH, Ostermayer SH, Büscheck F, Fischer E, Leetz M, Sievert H. PLAATO (percutaneous left atrial appendage transcatheter occlusion) for prevention of cardioembolic stroke in non-anticoagulation eligible atrial fibrillation patients: results from the European PLAATO study. EuroIntervention. 2010; 6:220–226.19. Freixa X, Tzikas A, Sobrino A, Chan J, Basmadjian AJ, Ibrahim R. Left atrial appendage closure with the Amplatzer™ Cardiac Plug: impact of shape and device sizing on follow-up leaks. Int J Cardiol. 2013; 168:1023–1027.20. Neuzner J, Dietze T, Paliege R, Möller M, Saeed G, Gradaus R. Left atrial appendage closure with the Amplatzer™ Cardiac Plug: Rationale for a higher degree of device oversizing at implantation. Cardiol J. 2015; 22:201–205.21. Otani K, Takeuchi M, Kaku K, Haruki N, Yoshitani H, Tamura M, Abe H, Okazaki M, Ota T, Lang RM, Otsuji Y. Impact of diastolic dysfunction grade on left atrial mechanics assessed by two-dimensional speckle tracking echocardiography. J Am Soc Echocardiogr. 2010; 23:961–967.22. Wong RC, Yeo TC. Left atrial volume is an independent predictor of exercise capacity in patients with isolated left ventricular diastolic dysfunction. Int J Cardiol. 2010; 144:425–427.23. Kizer JR, Bella JN, Palmieri V, Liu JE, Best LG, Lee ET, Roman MJ, Devereux RB. Left atrial diameter as an independent predictor of first clinical cardiovascular events in middle-aged and elderly adults: the Strong Heart Study (SHS). Am Heart J. 2006; 151:412–418.24. Tsang TS, Barnes ME, Gersh BJ, Takemoto Y, Rosales AG, Bailey KR, Seward JB. Prediction of risk for first age-related cardiovascular events in an elderly population: the incremental value of echocardiography. J Am Coll Cardiol. 2003; 42:1199–1205.25. Chinali M, de Simone G, Roman MJ, Bella JN, Liu JE, Lee ET, Best LG, Howard BV, Devereux RB. Left atrial systolic force and cardiovascular outcome. The Strong Heart Study. Am J Hypertens. 2005; 18(12 Pt 1):1570–1576.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Heart within a Heart
- Delayed Sealing of WATCHMAN Device Shunt
- A Comparative Study of Three Imaging Modalities for Size Selection of a Watchman Left Atrial Appendage Closure Device
- Subacute, Silent Embolization of Amplatzer Atrial Septal Defect Closure Device to the Pulmonary Artery
- Echocardiographic Evaluation of Atrial Septal Defect Device Closure