J Clin Neurol.
2010 Mar;6(1):46-50. 10.3988/jcn.2010.6.1.46.
A Case of Gerstmann-Straussler-Scheinker Disease
- Affiliations
-
- 1Department of Neurology, Bongseng Memorial Hospital, Busan, Korea.
- 2Department of Neurology, Wallace Memorial Baptist Hospital, Busan, Korea.
- 3Department of Neurology, Dong-A University College of Medicine, Busan, Korea. jwkim@dau.ac.kr
- 4Department of Radiology, Dong-A University College of Medicine, Busan, Korea.
- 5Department of Microbiology, Hallym University College of Medicine, Chuncheon, Korea.
- KMID: 2135482
- DOI: http://doi.org/10.3988/jcn.2010.6.1.46
Abstract
- BACKGROUND
Gerstmann-Straussler-Scheinker disease (GSS) is a type of human transmissible spongiform encephalopathy (TSE) that is determined genetically.
CASE REPORT
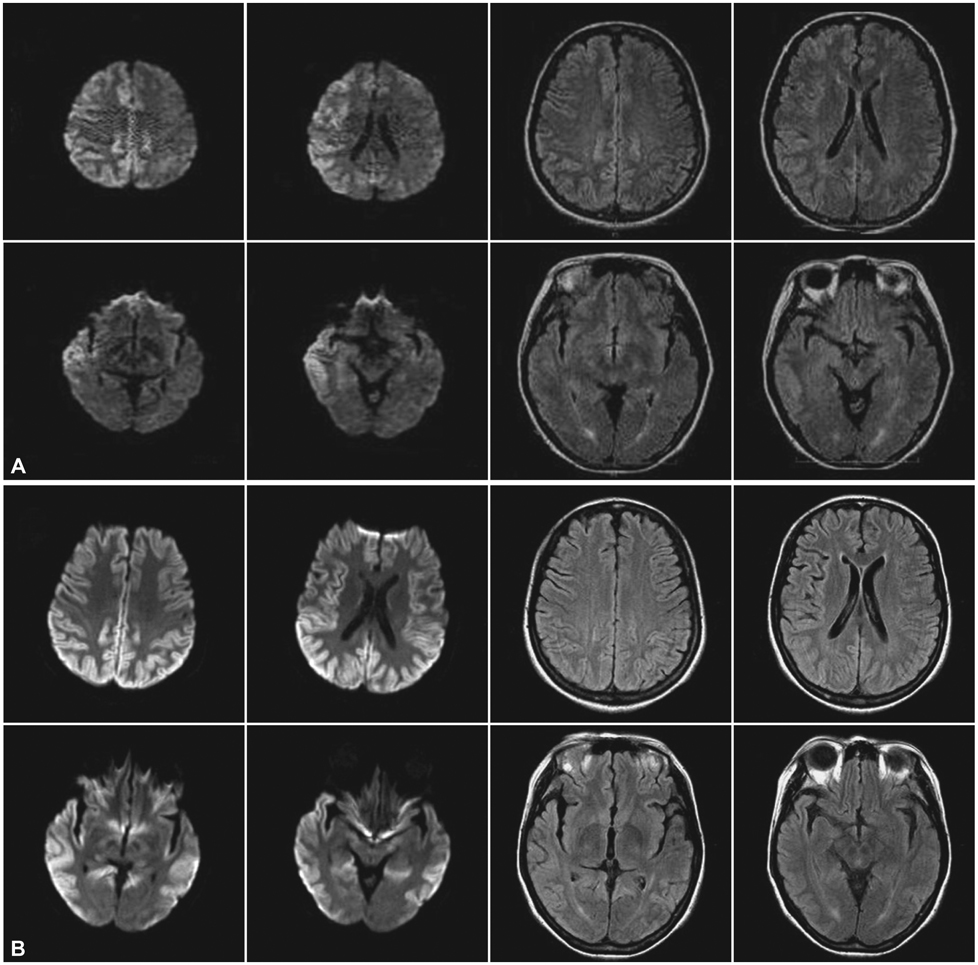
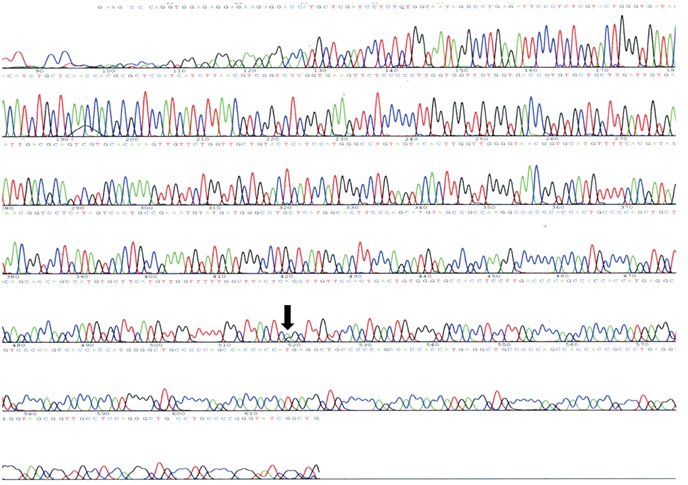
A 46-year-old woman presented with a slowly progressive ataxic gait and cognitive decline. She was alert but did not cooperate well due to severe dementia and dysarthria. High signal intensities in the cerebral cortices were evident in MRI, especially in diffusion-weighted images (DWI). A prion protein gene (PRNP) analysis revealed a P102L (proline-to-leucine) mutation in codon 102.
CONCLUSIONS
This is the first reported case of GSS (confirmed by PRNP analysis) in Korea. Distinctive MRI findings are also presented.
Keyword
MeSH Terms
Figure
Reference
-
1. Prusiner SB. Shattuck lecture-neurodegenerative diseases and prions. N Engl J Med. 2001. 344:1516–1526.2. Gerstmann J. Uber ein noch nicht beschriebenes Reflexphanomen bei einer Erkrankung des zerebellaren systems. Wein Medizin Wochenschr. 1928. 78:906–908.3. Gerstmann J, Sträussler E, Scheinker I. Uber eine eigenartige hereditar-familiare Erkrankung des Zentralnervensystems. Zugleich ein Beitrag zur Frage des vorzeitigen lokalen Alterns. Z Neurol. 1936. 154:736–762.
Article4. Hsiao K, Baker HF, Crow TJ, Poulter M, Owen F, Terwilliger JD, et al. Linkage of a prion protein missense variant to Germann-Sträsler syndrome. Nature. 1989. 338:342–345.
Article5. Arata H, Takashima H, Hirano R, Tomimitsu H, Machigashira K, Izumi K, et al. Early clinical signs and imaging findings in Gerstmann-Sträussler-Scheinker syndrome (Pro102Leu). Neurology. 2006. 66:1672–1678.
Article6. Collins SJ, Lawson VA, Masters CL. Transmissible spongiform encephalopathies. Lancet. 2004. 363:51–61.
Article7. Steinhoff BJ, Räker S, Herrendorf G, Poser S, Grosche S, Zerr I, et al. Accuracy and reliability of periodic sharp wave complexes in Creutzfeldt-Jakob disease. Arch Neurol. 1996. 53:162–166.
Article8. Zerr I, Bodemer M, Gefeller O, Otto M, Poser S, Wiltfang J, et al. Detection of 14-3-3 protein in the cerebrospinal fluid supports the diagnosis of Creutzfeldt-Jakob disease. Ann Neurol. 1998. 43:32–40.
Article9. Kovács GG, Puopolo M, Ladogana A, Pocchiari M, Budka H, van Duijn C, et al. Genetic prion disease: the EUROCJD experience. Hum Genet. 2005. 118:166–174.
Article10. Shiga Y, Miyazawa K, Sato S, Fukushima R, Shibuya S, Sato Y, et al. Diffusion-weighted MRI abnormalities as an early diagnostic marker for Creutzfeldt-Jakob disease. Neurology. 2004. 63:443–449.
Article11. Mittal S, Farmer P, Kalina P, Kingsley PB, Halperin J. Correlation of diffusion-weighted magnetic resonance imaging with neuropathology in Creutzfeldt-Jakob disease. Arch Neurol. 2002. 59:128–134.
Article12. Takase K, Furuya H, Murai H, Yamada T, Oh-yagi Y, Doh-ura K, et al. A case of Gerstmann-Sträussler-Scheinker syndrome (GSS) with late onset-a haplotype analysis of Glu219Lys polymorphism in PrP gene. Rinsho Shinkeigaku. 2001. 41:318–321.13. Misumi M, Nishida Y, Araki S. Patient with Gerstmann-Sträussler-Scheinker syndrome (GSS P102L) presenting high intensity lesions in the cerebral cortex on diffusion weighted MRI. Rinsho Shinkeigaku. 2006. 46:291–293.14. Irisawa M, Amanuma M, Kozawa E, Kimura F, Araki N. A case of Gerstmann-Sträsler-Scheinker syndrome. Magn Reson Med Sci. 2007. 6:53–57.15. Konaka K, Kaido M, Okuda Y, Aoike F, Abe K, Kitamoto T, et al. Proton magnetic resonance spectroscopy of a patient with Gerstmann-Sträussler-Scheinker disease. Neuroradiology. 2000. 42:662–665.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gerstmann-Sträussler-Scheinker Disease (Pro102Leu) Presenting as Rapidly Progressive Dementia
- Gerstmann-SträusslerScheinker Disease: A Case Report
- Accumulation Area of a Japanese PRNP P102L Variant Associated With Gerstmann–Sträussler–Scheinker Disease: The Ariake PRNP P102L Variant
- The Homogeneity of Phenomenology of Gerstmann Syndrome: The Study in Patients with Alzheimer's Disease
- Characteristics of Genetic Creutzfeldt-Jakob Disease in Korea: 2017-2023