J Bacteriol Virol.
2014 Mar;44(1):52-58. 10.4167/jbv.2014.44.1.52.
Evaluation of Immune Response for Vi-CRM(197) Conjugated Vaccine against Salmonella enterica serovar Typhi in Mice
- Affiliations
-
- 1College of Veterinary Medicine & Institute of Veterinary Science, Kangwon National University, Chuncheon, Korea. twhahn@kangwon.ac.kr
- 2Eubiologics Co., Ltd., Soyang-ro 56, Chuncheon, Korea.
- KMID: 2135357
- DOI: http://doi.org/10.4167/jbv.2014.44.1.52
Abstract
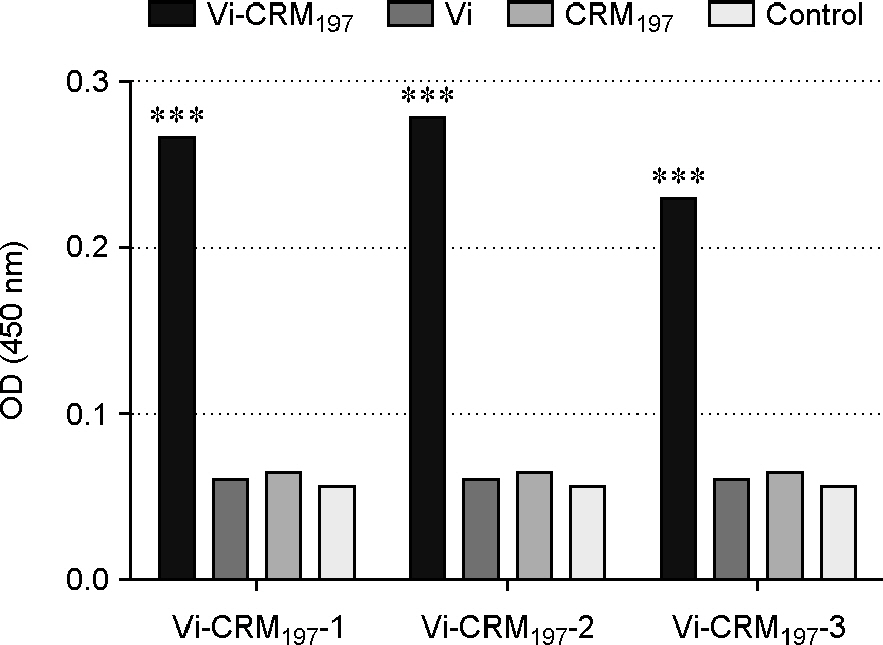
- Typhoid fever, a serious systemic infection caused by Salmonella enterica serovar Typhi, breaks out in developing countries. However, existing vaccines only induce relatively low protective effects with humoral responses and do not stimulate secondary immune response, especially to young people. The objective of this study is to evaluate the immunogenicity of the vaccine containing virulence capsular polysaccharide (Vi) conjugated with the optimal ratios of non-toxic variant of diphtheria toxin (CRM(197)) in mice. Six-week-old BALB/c female mice were injected intraperitoneally three times at intervals of 14 days and sera were collected on days 0, 14, 28, 42 and 56 post-injection. The efficacy of the vaccine was evaluated by comparing between negative control group injected with PBS and vaccine groups injected with Vi or Vi-CRM(197) conjugate of different ratio. Vi and CRM(197)-specific antibody responses were evaluated using enzyme-linked immunosorbent assay. The result showed that Vi-CRM(197)-1 group revealed the highest and significant Vi-specific IgG immune responses among the other groups and Vi group (p < 0.01). In conclusion, Vi-CRM(197)-1 conjugate vaccine induced the highest humoral immune response in mice and may be used as an effective vaccine to replace the existing typhoid vaccine for infants under 2 years old.
Keyword
MeSH Terms
-
Animals
Antibody Formation
Child, Preschool
Developing Countries
Diphtheria Toxin
Enzyme-Linked Immunosorbent Assay
Female
Humans
Immunity, Humoral
Immunoglobulin G
Infant
Mice*
Salmonella enterica*
Salmonella typhi*
Salmonella*
Typhoid Fever
Typhoid-Paratyphoid Vaccines
Vaccines
Virulence
Diphtheria Toxin
Immunoglobulin G
Typhoid-Paratyphoid Vaccines
Vaccines
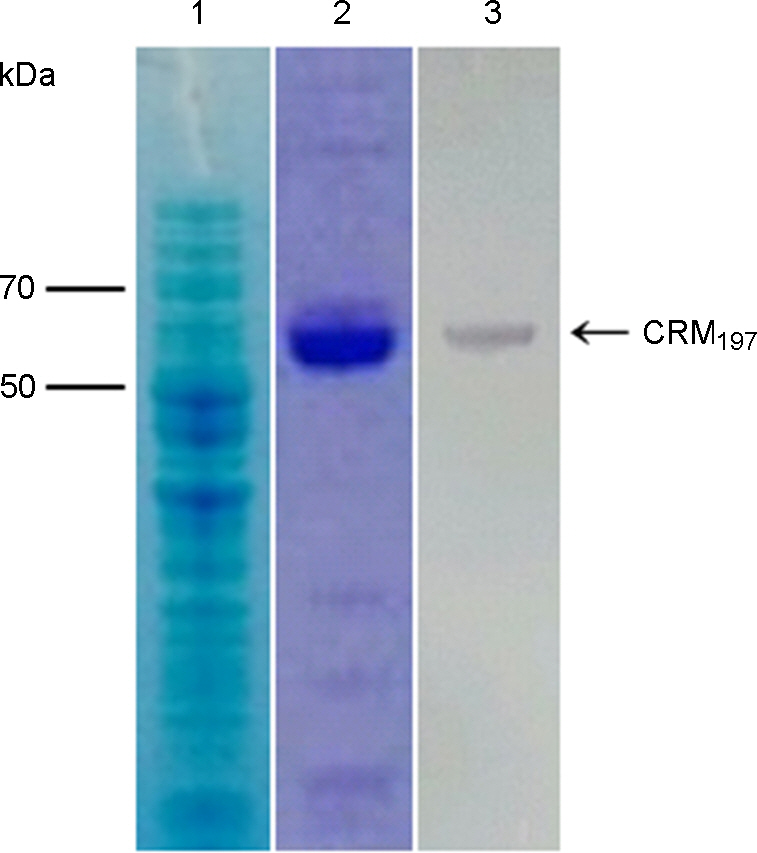
Figure
Cited by 1 articles
-
Application of radiation technology in vaccines development
Ho Seong Seo
Clin Exp Vaccine Res. 2015;4(2):145-158. doi: 10.7774/cevr.2015.4.2.145.
Reference
-
1). Cui C, Carbis R, An SJ, Jang H, Czerkinsky C, Szu SC, et al. Physical and chemical characterization and immunologic properties of Salmonella enterica serovar typhi capsular polysaccharide-diphtheria toxoid conjugates. Clin Vaccine Immunol. 2010; 17:73–9.2). Toobak H, Rasooli I, Talei D, Jahangiri A, Owlia P, Darvish Alipour Astaneh S. Immune response variations to Salmonella enterica serovar Typhi recombinant porin proteins in mice. Biologicals. 2013; 41:224–30.3). Lu YJ, Zhang F, Sayeed S, Thompson CM, Szu S, Anderson PW, et al. A bivalent vaccine to protect against Streptococcus pneumoniae and Salmonella typhi. Vaccine. 2012; 30:3405–12.4). No authors listed. Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008; 83:49–59.5). Marathe SA, Lahiri A, Negi VD, Chakravortty D. Typhoid fever & vaccine development: a partially answered question. Indian J Med Res. 2012; 135:161–9.6). An SJ, Yoon YK, Kothari S, Kim DR, Kim JA, Kothari N, et al. Immune suppression induced by Vi capsular polysaccharide is overcome by Vi-DT conjugate vaccine. Vaccine. 2012; 30:1023–8.
Article7). Guzman CA, Borsutzky S, Griot-Wenk M, Metcalfe IC, Pearman J, Collioud A, et al. Vaccines against typhoid fever. Vaccine. 2006; 24:3804–11.
Article8). DeRoeck D, Ochiai RL, Yang J, Anh DD, Alag V, Clemens JD. Typhoid vaccination: the Asian experience. Expert Rev Vaccines. 2008; 7:547–60.
Article9). An SJ, Yoon YK, Kothari S, Kothari N, Kim JA, Lee E, et al. Physico-chemical properties of Salmonella typhi Vi polysaccharide-diphtheria toxoid conjugate vaccines affect immunogenicity. Vaccine. 2011; 29:7618–23.10). Jones C. Vaccines based on the cell surface carbohydrates of pathogenic bacteria. An Acad Bras Cienc. 2005; 77:293–324.
Article11). Croxtall JD, Keating GM. Pneumococcal polysaccharide protein D-conjugate vaccine (Synflorix; PHiD-CV). Paediatr Drugs. 2009; 11:349–57.
Article12). Micoli F, Rondini S, Pisoni I, Proietti D, Berti F, Costantino P, et al. Vi-CRM197 as a new conjugate vaccine against Salmonella Typhi. Vaccine. 2011; 29:712–20.13). Snape MD, Perrett KP, Ford KJ, John TM, Pace D, Yu LM, et al. Immunogenicity of a tetravalent meningococcal glycoconjugate vaccine in infants: a randomized controlled trial. JAMA. 2008; 299:173–84.
Article14). Jackson LA, Jacobson RM, Reisinger KS, Anemona A, Danzig LE, Dull PM. A randomized trial to determine the tolerability and immunogenicity of a quadrivalent meningococcal glycoconjugate vaccine in healthy adolescents. Pediatr Infect Dis J. 2009; 28:86–91.
Article15). Shinefield HR. Overview of the development and current use of CRM197 conjugate vaccines for pediatric use. Vaccine. 2010; 28:4335–9.16). Rondini S, Micoli F, Lanzilao L, Pisoni I, Di Cioccio V, Saul AJ, et al. Characterization of Citrobacter sp. line 328 as a source of Vi for a Vi-CRM197 glycoconjugate vaccine against Salmonella Typhi. J Infect Dev Ctries. 2012; 6:763–73.17). Jang H, Yoon YK, Kim JA, Kim HS, An SJ, Seo JH, et al. Optimization of Vi capsular polysaccharide production during growth of Salmonella enterica serotype Typhi Ty2 in a bioreactor. J Biotechnol. 2008; 135:71–7.18). Santander J, Roland KL, Curtiss R 3rd. Regulation of Vi capsular polysaccharide synthesis in Salmonella enterica serotype Typhi. J Infect Dev Ctries. 2008; 2:412–20.
Article19). Fiorino F, Ciabattini A, Rondini S, Pozzi G, Martin LB, Medaglini D. Immunization with the conjugate vaccine Vi-CRM197 against Salmonella Typhi Induces Vi-specific mucosal and systemic immune responses in mice. Vaccine. 2012; 30:6111–4.20). Finn A. Bacterial polysaccharide-protein conjugate vaccines. Br Med Bull. 2004; 70:1–14.
Article21). Vliegenthart JF. Carbohydrate based vaccines. FEBS Lett. 2006; 580:2945–50.
Article22). van Damme P, Kafeja F, Anemona A, Basile V, Hilbert AK, De Coster I, et al. Safety, immunogenicity and dose ranging of a new Vi-CRM197 conjugate vaccine against typhoid fever: randomized clinical testing in healthy adults. PLoS One. 2011; 6:e25398.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of Salmonella enterica serovar Typhi endocarditis with multiple splenic infarctions
- Salmonella Serovars from Foodborne and Waterborne Diseases in Korea, 1998-2007: Total Isolates Decreasing Versus Rare Serovars Emerging
- Genomic Characteristics and Identification of Salmonella enterica serovars Typhi and Paratyphi A Using Multiplex PCR
- Modulation of Humoral and Cell-Mediated Immunity Against Avian Influenza and Newcastle Disease Vaccines by Oral Administration of Salmonella enterica Serovar Typhimurium Expressing Chicken Interleukin-18
- A Case of Typhoid Fever to Failed Ciprofloxacin, Infected in Korea