J Korean Ophthalmol Soc.
2015 May;56(5):721-726. 10.3341/jkos.2015.56.5.721.
Intravitreal Injection of Dexamethasone Implant during Cataract Surgery in Patients with Noninfectious Uveitis
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University College of Medicine, Seoul, Korea. hjw68@snu.ac.kr
- 2Department of Ophthalmology, Chuncheon Sacred Heart Hospital, Hallym University College of Medicine, Chuncheon, Korea.
- KMID: 2121170
- DOI: http://doi.org/10.3341/jkos.2015.56.5.721
Abstract
- PURPOSE
To investigate the efficacy and safety of intravitreal dexamethasone implant for controlling postoperative inflammation among uveitis patients undergoing cataract extraction.
METHODS
Ten eyes with noninfectious uveitis underwent phacoemulsification with intraocular lens implantation followed by intravitreal injection of 0.7-mg dexamethasone implant (implant group) between February 2011 and January 2014. Twenty age- and gender-matched controls who received cataract surgery without implantation during the same period were recruited (non-implant group). Medical records of the subjects were retrospectively reviewed and 6-month postoperative clinical outcomes were compared between the 2 groups.
RESULTS
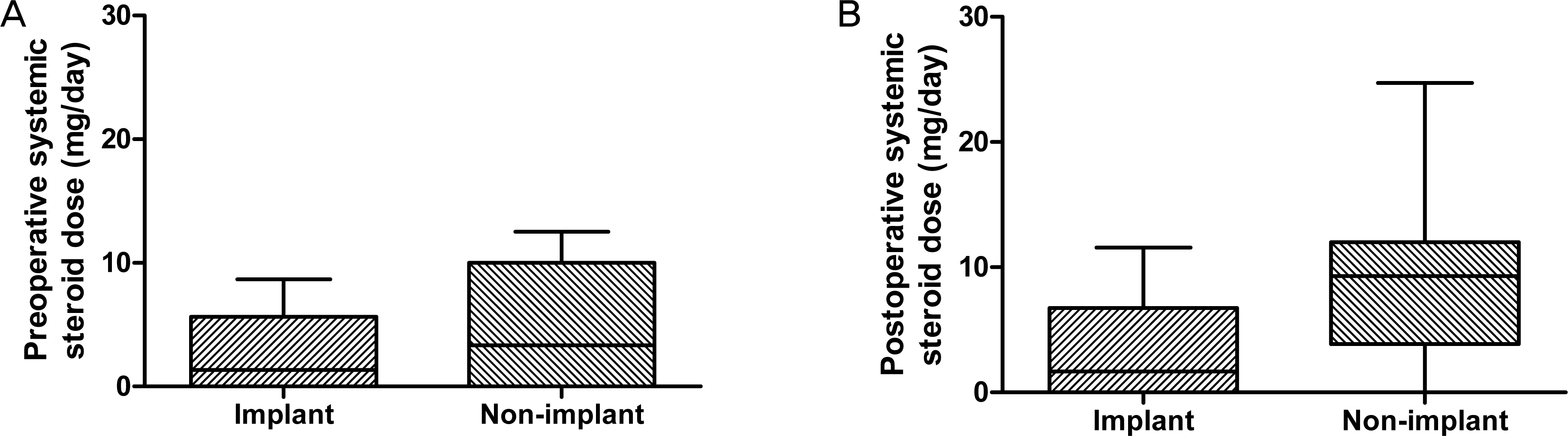
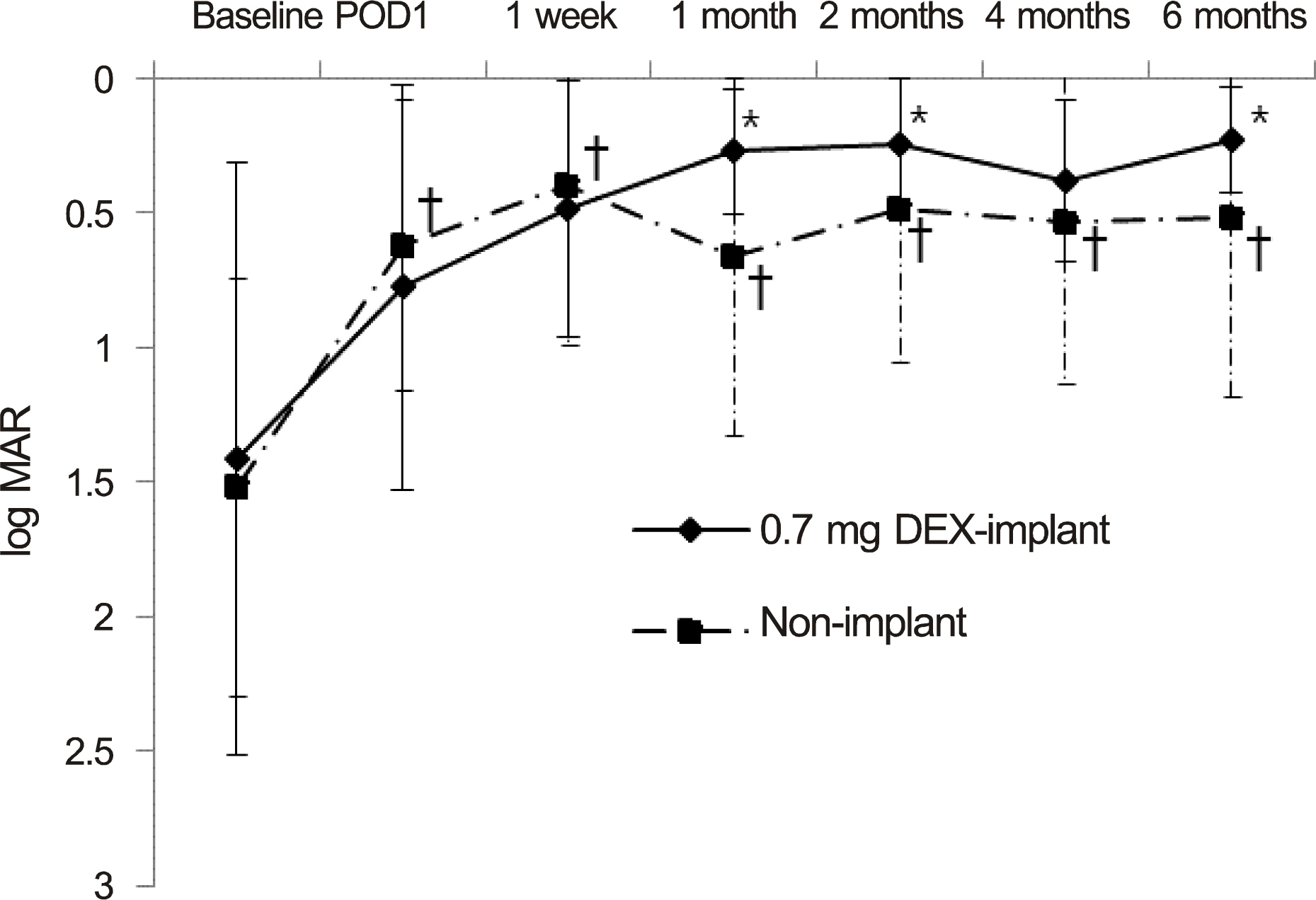
The mean age was 42.30 +/- 15.81 years in the implant group and 45.65 +/- 13.63 years in the non-implant group. The 2 groups were similar in terms of age, gender, preoperative inflammatory status, and preoperative visual acuity (p = 0.552, 1.000, 0.133 and 0.767, respectively). After surgery, oral steroid was used in the non-implant group (8.8 +/- 1.5 mg/day on average) and the implant group (3.5 +/- 1.3 mg/day; p = 0.029). Visual acuity (log MAR) improved significantly in both groups (p = 0.789) with no significant difference between the 2 groups. Postoperative recurrence rates of uveitis were reduced more (40%) in the implant group than in the non-implant group (50%) but without significance (p = 0.709). Elevated intraocular pressure > or =25 mm Hg occurred in 3 eyes (30%) in the implant group and 4 eyes (20%) in the non-implant group (p = 0.657), of which 1 in each group required a filtering surgery. Otherwise, no significant complications developed in either group.
CONCLUSIONS
Intravitreal dexamethasone implants help reduce conventional oral steroid dosage for controlling postoperative inflammation. Dexamethasone implants could be an effective and safe alternative to control the inflammation after cataract surgery in uveitis patients.
MeSH Terms
Figure
Reference
-
References
1. Kim JC, Ham DI. Clinical characteristics and treatments of intermediate uveitis. J Korean Ophthalmol Soc. 2009; 50:85–91.
Article2. Foster CS, Fong LP, Singh G. Cataract surgery and intraocular lens implantation in patients with uveitis. Ophthalmology. 1989; 96:281–8.
Article3. Krishna R, Meisler DM, Lowder CY, et al. Long-term follow-up of extracapsular cataract extraction and posterior chamber intraocular lens implantation in patients with uveitis. Ophthalmology. 1998; 105:1765–9.
Article4. Okhravi N, Lightman SL, Towler HM. Assessment of visual outcome after cataract surgery in patients with uveitis. Ophthalmology. 1999; 106:710–22.
Article5. Kim YW, Seo KY. The Results of Phacoemulsification Cataract Surgery in Patients with Behcet's Disease. J Korean Ophthalmol Soc. 2006; 47:1943–7.6. Estafanous MF, Lowder CY, Meisler DM, Chauhan R. Phacoemulsification cataract extraction and posterior chamber lens implantation in patients with uveitis. Am J Ophthalmol. 2001; 131:620–5.
Article7. Ram J, Gupta A, Kumar S, et al. Phacoemulsification with intraocular lens implantation in patients with uveitis. J Cataract Refract Surg. 2010; 36:1283–8.
Article8. Kosker M, Sungur G, Celik T, et al. Phacoemulsification with intraocular lens implantation in patients with anterior uveitis. J Cataract Refract Surg. 2013; 39:1002–7.
Article9. Laurell CG, Zetterström C. Effects of dexamethasone, diclofenac, or placebo on the inflammatory response after cataract surgery. Br J Ophthalmol. 2002; 86:1380–4.
Article10. Foster CS, Rashid S. Management of coincident cataract and uveitis. Curr Opin Ophthalmol. 2003; 14:1–6.
Article11. Han HC, Bang JW, Yum JH, et al. A case of acute endophthalmitis following a dexamethasone intravitreal implant. J Korean Ophthalmol Soc. 2013; 54:1939–44.
Article12. Chang-Lin JE, Attar M, Acheampong AA, et al. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011; 52:80–6.
Article13. Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997; 13:388–91.
Article14. Jabs DA, Nussenblatt RB, Rosenbaum JT; Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data. Results of the First International Workshop. Am J Ophthalmol. 2005; 140:509–16.15. Okhravi N, Morris A, Kok HS, et al. Intraoperative use of intravitreal triamcinolone in uveitic eyes having cataract surgery: pilot study. J Cataract Refract Surg. 2007; 33:1278–83.
Article16. Dada T, Dhawan M, Garg S, et al. Safety and efficacy of intraoperative intravitreal injection of triamcinolone acetonide injection after phacoemulsification in cases of uveitic cataract. J Cataract Refract Surg. 2007; 33:1613–8.
Article17. Paganelli F, Cardillo JA, Melo LA Jr, et al. A single intraoperative subTenon's capsule triamcinolone acetonide injection for the treatment of post-cataract surgery inflammation. Ophthalmology. 2004; 111:2102–8.
Article18. Chang DT, Herceg MC, Bilonick RA, et al. Intracameral dexamethasone reduces inflammation on the first postoperative day after cataract surgery in eyes with and without glaucoma. Clin Ophthalmol. 2009; 3:345–55.
Article19. Chieh JJ, Carlson AN, Jaffe GJ. Combined fluocinolone acetonide intraocular delivery system insertion, phacoemulsification, and intraocular lens implantation for severe uveitis. Am J Ophthalmol. 2008; 146:589–94.
Article20. Gupta A, Ram J, Gupta A, Gupta V. Intraoperative dexamethasone implant in uveitis patients with cataract undergoing phacoemulsification. Ocul Immunol Inflamm. 2013; 21:462–7.
Article21. van Kooij B, Rothova A, de Vries P. The pros and cons of intravitreal triamcinolone injections for uveitis and inflammatory cystoid macular edema. Ocul Immunol Inflamm. 2006; 14:73–85.
Article22. Ram J, Kaushik S, Brar GS, et al. Phacoemulsification in patients with Fuchs' heterochromic uveitis. J Cataract Refract Surg. 2002; 28:1372–8.
Article23. Kawaguchi T, Mochizuki M, Miyata K, Miyata N. Phacoemulsification cataract extraction and intraocular lens implantation in patients with uveitis. J Cataract Refract Surg. 2007; 33:305–9.
Article24. Rahman I, Jones NP. Long-term results of cataract extraction with intraocular lens implantation in patients with uveitis. Eye (Lond). 2005; 19:191–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Therapeutic Efficacy of Intravitreal Dexamethasone Implant in Korean Patients with Non-infectious Uveitis
- Effect of Intravitreal Dexamethasone Implant Injection in a Patient with Recurrent Nodular Anterior Scleritis
- A Case of Retinal Hemorrhage Following a Dexamethasone Intravitreal Implant
- A Case of Cytomegalovirus Retinitis Following Intravitreal Dexamethasone Implant in an Immunocompetent Patient with Uveitis
- Surgical Management of Complications after Dexamethasone Implant Injection