Clin Exp Otorhinolaryngol.
2015 Sep;8(3):268-274. 10.3342/ceo.2015.8.3.268.
A Higher Angiogenin Expression is Associated With a Nonnuclear Maspin Location in Laryngeal Carcinoma
- Affiliations
-
- 1Department of Neurosciences, Otolaryngology Section, University of Padova, Padova, Italy. andrea.lovato.3@hotmail.it
- 2Department of Medicine, University of Padova, Padova, Italy.
- 3Department of Neurosciences, Otolaryngology Section, Treviso Hospital Branch, University of Padova, Treviso, Italy.
- KMID: 2117523
- DOI: http://doi.org/10.3342/ceo.2015.8.3.268
Abstract
OBJECTIVES
In numerous malignancies, angiogenin (ANG) and Maspin are important proangiogenic and antiangiogenic regulators, respectively. The aim of this study was to identify potential relationships between the biological roles of these two proteins in laryngeal squamous cell carcinoma (LSCC).
METHODS
Immunohistochemical staining for ANG and Maspin was performed on specimens from 76 consecutive LSCC patients treated with surgery alone, considering the subcellular pattern of Maspin expression. Univariate and multivariate statistical models were used for prognostic purposes.
RESULTS
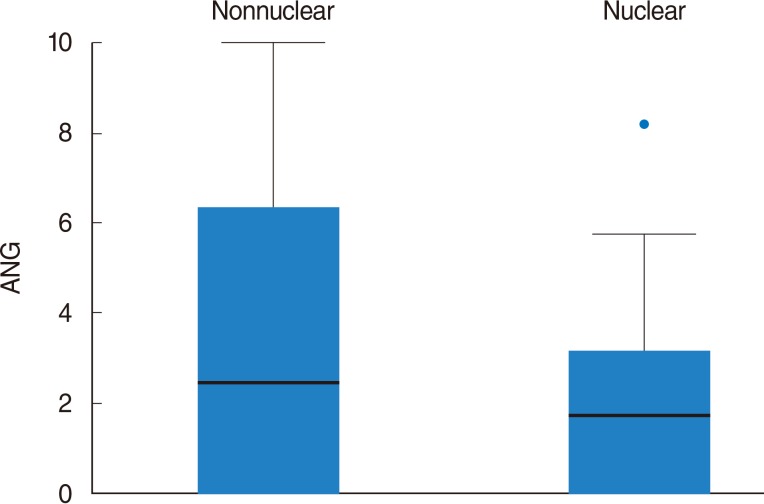
On univariate analysis, a different level of ANG expression was seen for patients stratified by subcellular Maspin expression pattern: the mean ANG expression was higher in cases with a nonnuclear MASPIN expression than in those with a nuclear pattern (P=0.002). Disease-free survival (DFS; in months) differed significantly when patients were stratified by N stage (P=0.01). Patients whose Maspin expression was nonnuclear (i.e., it was cytoplasmic or there was none) had a significantly higher recurrence rate (P<0.001), and shorter DFS (P=0.01) than those with a nuclear Maspin pattern. The mean ANG expression was significantly higher in cases with loco-regional recurrent disease (P=0.007); and patients with an ANG expression > or =5.0% had a significantly shorter DFS than those with an ANG expression <5.0% (P=0.007). On multivariate analysis, ANG expression > or =5.0% was a significant, independent, negative prognostic factor in terms of DFS (P=0.041).
CONCLUSION
Our results support the hypothesis that a higher ANG expression is associated with a nonnuclear Maspin expression pattern in patients with LSCC. Further studies are needed to clarify the relationship between the ANG and Maspin pathways, and their potential diagnostic and therapeutic role in LSCC.
MeSH Terms
Figure
Reference
-
1. Tello-Montoliu A, Patel JV, Lip GY. Angiogenin: a review of the pathophysiology and potential clinical applications. J Thromb Haemost. 2006; 9. 4(9):1864–1874. PMID: 16961595.
Article2. Yoshioka N, Wang L, Kishimoto K, Tsuji T, Hu GF. A therapeutic target for prostate cancer based on angiogenin-stimulated angiogenesis and cancer cell proliferation. Proc Natl Acad Sci U S A. 2006; 9. 103(39):14519–14524. PMID: 16971483.
Article3. Kishimoto K, Liu S, Tsuji T, Olson KA, Hu GF. Endogenous angiogenin in endothelial cells is a general requirement for cell proliferation and angiogenesis. Oncogene. 2005; 1. 24(3):445–456. PMID: 15558023.
Article4. Gao X, Xu Z. Mechanisms of action of angiogenin. Acta Biochim Biophys Sin (Shanghai). 008; 7. 40(7):619–624. PMID: 18604453.5. Li Z, Shi HY, Zhang M. Targeted expression of maspin in tumor vasculatures induces endothelial cell apoptosis. Oncogene. 2005; 3. 24(12):2008–2019. PMID: 15688005.
Article6. Bodenstine TM, Seftor RE, Khalkhali-Ellis Z, Seftor EA, Pemberton PA, Hendrix MJ. Maspin: molecular mechanisms and therapeutic implications. Cancer Metastasis Rev. 2012; 12. 31(3-4):529–551. PMID: 22752408.
Article7. Marioni G, Staffieri A, Blandamura S. Maspin expression in head and neck carcinoma: subcellular localization matters. J Oral Pathol Med. 2010; 3. 39(3):279–280. PMID: 20141575.
Article8. Gurzu S, Szentirmay Z, Jung I. Molecular classification of colorectal cancer: a dream that can become a reality. Rom J Morphol Embryol. 2013; 54(2):241–245. PMID: 23771065.9. Marioni G, Giacomelli L, D'Alessandro E, Staffieri C, Guzzardo V, Staffieri A, et al. Laryngeal carcinoma recurrence rate and disease-free interval are related to CD105 expression but not to vascular endothelial growth factor 2 (Flk-1/Kdr) expression. Anticancer Res. 2008; Jan-Feb. 28(1B):551–557. PMID: 18383901.10. Marioni G, Marino F, Blandamura S, D'Alessandro E, Giacomelli L, Guzzardo V, et al. Neoangiogenesis in laryngeal carcinoma: angiogenin and CD105 expression is related to carcinoma recurrence rate and disease-free survival. Histopathology. 2010; 10. 57(4):535–543. PMID: 20955379.
Article11. Marioni G, D'Alessandro E, Giacomelli L, De Filippis C, Calgaro N, Sari M, et al. Maspin nuclear localization is related to reduced density of tumour-associated micro-vessels in laryngeal carcinoma. Anticancer Res. 2006; Nov-Dec. 26(6C):4927–4932. PMID: 17214364.12. Sobin LH, Gospodarowicz MK, Wittekind C. TNM classification of malignant tumors. 7th ed. Oxford: Wiley-Blackwell;2009.13. Marioni G, Marchese-Ragona R, Cartei G, Marchese F, Staffieri A. Current opinion in diagnosis and treatment of laryngeal carcinoma. Cancer Treat Rev. 2006; 11. 32(7):504–515. PMID: 16920269.
Article14. Marioni G, Staffieri A, Bertolin A, Giacomelli L, D'Alessandro E, Ottaviano G, et al. Laryngeal carcinoma lymph node metastasis and disease-free survival correlate with MASPIN nuclear expression but not with EGFR expression: a series of 108 cases. Eur Arch Otorhinolaryngol. 2010; 7. 267(7):1103–1110. PMID: 20052590.
Article15. Howlader N, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, editors. SEER cancer statistics review, 1975-2010 [Internet]. Bethesda (MD): National Cancer Institute;2013. cited 2013 Jun 30. Available from: http://seer.cancer.gov/csr/1975_2010/.16. Vlachtsis K, Nikolaou A, Markou K, Fountzilas G, Daniilidis I. Clinical and molecular prognostic factors in operable laryngeal cancer. Eur Arch Otorhinolaryngol. 2005; 11. 262(11):890–898. PMID: 15739081.
Article17. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; Jan-Feb. 62(1):10–29. PMID: 22237781.
Article18. Marioni G, Blandamura S, Lionello M, Giacomelli L, Lovato A, Favaretto N, et al. Indications for postoperative radiotherapy in laryngeal carcinoma: a panel of tumor tissue markers for predicting locoregional recurrence in surgically treated carcinoma: a pilot study. Head Neck. 2014; 11. 36(11):1534–1540. PMID: 23996283.
Article19. Marioni G, Blandamura S, Loreggian L, Koussis H, Lionello M, Giacomelli L, et al. Laryngeal carcinoma prognosis after postoperative radiotherapy correlates with CD105 expression, but not with angiogenin or EGFR expression. Eur Arch Otorhinolaryngol. 2011; 12. 268(12):1779–1787. PMID: 21842202.
Article20. Marioni G, Giacomelli L, D'Alessandro E, Marchese-Ragona R, Staffieri C, Ferraro SM, et al. Nuclear localization of mammary serine protease inhibitor (MASPIN): is its impact on the prognosis in laryngeal carcinoma due to a proapoptotic effect? Am J Otolaryngol. 2008; May-Jun. 29(3):156–162. PMID: 18439947.
Article21. Marioni G, Koussis H, Scola A, Maruzzo M, Giacomelli L, Karahontziti P, et al. Expression of MASPIN and angiogenin in nasopharyngeal carcinoma: novel preliminary clinico-pathological evidence. Acta Otolaryngol. 2010; 8. 130(8):952–958. PMID: 20105109.
Article22. Li S, Yu W, Hu GF. Angiogenin inhibits nuclear translocation of apoptosis inducing factor in a Bcl-2-dependent manner. J Cell Physiol. 2012; 4. 227(4):1639–1644. PMID: 21678416.
Article23. Sadagopan S, Veettil MV, Chakraborty S, Sharma-Walia N, Paudel N, Bottero V, et al. Angiogenin functionally interacts with p53 and regulates p53-mediated apoptosis and cell survival. Oncogene. 2012; 11. 31(46):4835–4847. PMID: 22266868.
Article24. Zou Z, Gao C, Nagaich AK, Connell T, Saito S, Moul JW, et al. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem. 2000; 3. 275(9):6051–6054. PMID: 10692390.
Article25. Zhang W, Zhang M. Tissue microarray analysis of maspin expression and its reverse correlation with mutant p53 in various tumors. Int J Oncol. 2002; 6. 20(6):1145–1150. PMID: 12011991.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinicopathological Significance of Maspin Expression in Breast Cancer
- Expression of Maspin in Squamous Cell Carcinoma of the Head and Neck
- Expression of beta-catenin in Hepatocellular Carcinoma in Relation to Tumor Cell Proliferation and Cyclin D1 Expression
- Expression of Maspin Protein in Ductal Hyperplasia, Intraductal Carcinoma and Invasive Ductal Carcinoma of the Breast
- Prognostic Significance of Maspin in Pancreatic Ductal Adenocarcinoma