Clin Exp Otorhinolaryngol.
2012 Jun;5(2):62-67. 10.3342/ceo.2012.5.2.62.
Galectin-1, -3, -7 Expressions in Congenital and Acquired Pediatric Cholesteatomas Compared to External Auditory Canal Skin
- Affiliations
-
- 1Department of Otorhinolaryngology, Erasmus University Hospital, Universite Libre de Bruxelles, Brussels, Belgium. mvdghins@ulb.ac.be
- 2Department of Pathology, Erasmus University Hospital, Universite Libre de Bruxelles, Brussels, Belgium.
- 3Department of Otorhinolaryngology, Reine Fabiola Children's Hospital, Brussels, Belgium.
- KMID: 2117498
- DOI: http://doi.org/10.3342/ceo.2012.5.2.62
Abstract
OBJECTIVES
There is a classical distinction based on clinical criteria between acquired and congenital cholesteatomas. To determine if these two types of lesions show different immunohistochemical features, we have studied the expression patterns of three distinctive galectins (animal lectins implied especially in cellular proliferation and apoptosis) in both types of cholesteatomas and compared it to their expression patterns in external auditory canal skin.
METHODS
Our study is based on nine acquired and eight congenital cholesteatomas, obtained from children during ear surgery. Six specimens of normal adult auditory meatal skin served as control. Specimens were analyzed by immunohistochemistry using monoclonal antibodies with galectin-1 and galectin-3, and a polyclonal antibody with galectin-7.
RESULTS
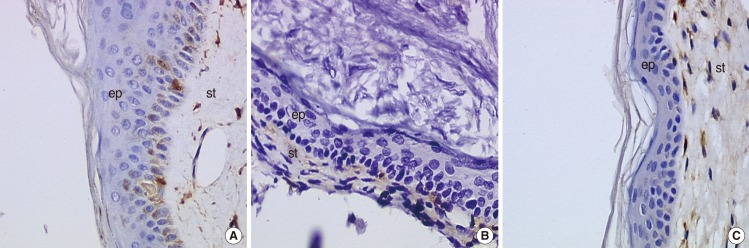
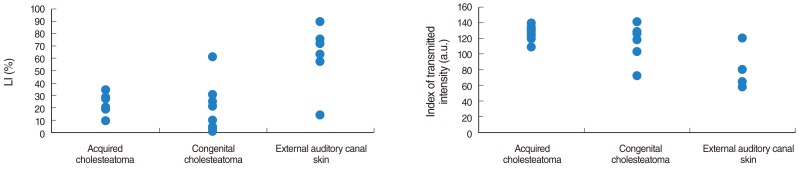
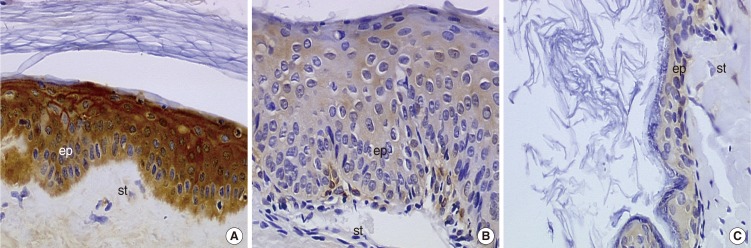
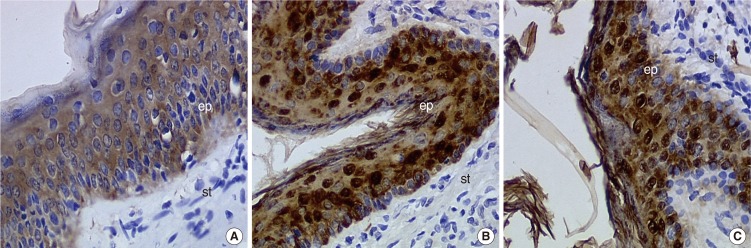
We did not observe any differences in the galectin distribution pattern between congenital and acquired pediatric cholesteatomas. Compared to the control group, cholesteatomas present some particular features. There was no expression of galectin-1 and a lower expression of galectin-3 in the epithelium. Furthermore, we observed a preferentially nuclear distribution of galectin-7 in cholesteatomas, whereas it is essentially cytoplasmic in the control group.
CONCLUSION
The data reported in this study suggest, on the basis of a lesser marked galectin-3 in cholesteatomas epithelium compared with an external auditory canal skin, that an immature keratinocytes population is at the origin of these lesions and that galectin-3 and galectin-7 play a part in the capacity as apoptosis modulators. Our study does not establish a difference in the galectin expressions of congenital and acquired cholesteatomas, but it constitutes however an additional argument in favor of the "undifferentiated" origin of keratinocytes in cholesteatomas.
MeSH Terms
Figure
Reference
-
1. Levenson MJ, Michaels L, Parisier SC, Juarbe C. Congenital cholesteatomas in children: an embryologic correlation. Laryngoscope. 1988; 9. 98(9):949–955. PMID: 3412093.2. Olszewska E, Wagner M, Bernal-Sprekelsen M, Ebmeyer J, Dazert S, Hildmann H, et al. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol. 2004; 1. 261(1):6–24. PMID: 12835944.
Article3. Tos M. A new pathogenesis of mesotympanic (congenital) cholesteatoma. Laryngoscope. 2000; 11. 110(11):1890–1897. PMID: 11081605.
Article4. Broekaert D. The problem of middle ear cholesteatoma: etiology, genesis and pathobiology - a review. Acta Otorhinolaryngol Belg. 1991; 45(4):355–367. PMID: 1767665.5. Choufani G, Mahillon V, Decaestecker C, Lequeux T, Danguy A, Salmon I, et al. Determination of the levels of expression of sarcolectin and calcyclin and of the percentages of apoptotic but not proliferating cells to enable distinction between recurrent and nonrecurrent cholesteatomas. Laryngoscope. 1999; 11. 109(11):1825–1831. PMID: 10569415.
Article6. Sheikholeslam-Zadeh R, Decaestecker C, Delbrouck C, Danguy A, Salmon I, Zick Y, et al. The levels of expression of galectin-3, but not of galectin-1 and galectin-8, correlate with apoptosis in human cholesteatomas. Laryngoscope. 2001; 6. 111(6):1042–1047. PMID: 11404618.
Article7. Perillo NL, Marcus ME, Baum LG. Galectins: versatile modulators of cell adhesion, cell proliferation, and cell death. J Mol Med (Berl). 1998; 5. 76(6):402–412. PMID: 9625297.
Article8. Elola MT, Chiesa ME, Alberti AF, Mordoh J, Fink NE. Galectin-1 receptors in different cell types. J Biomed Sci. 2005; 12(1):13–29. PMID: 15864736.
Article9. Scott K, Weinberg C. Galectin-1: a bifunctional regulator of cellular proliferation. Glycoconj J. 2004; 19(7-9):467–477. PMID: 14758070.
Article10. Dumic J, Dabelic S, Flogel M. Galectin-3: an open-ended story. Biochim Biophys Acta. 2006; 4. 1760(4):616–635. PMID: 16478649.
Article11. Liu FT, Patterson RJ, Wang JL. Intracellular functions of galectins. Biochim Biophys Acta. 2002; 9. 1572(2-3):263–273. PMID: 12223274.
Article12. Ochieng J, Furtak V, Lukyanov P. Extracellular functions of galectin-3. Glycoconj J. 2004; 19(7-9):527–535. PMID: 14758076.
Article13. Pelc P, Vanmuylder N, Lefranc F, Heizmann CW, Hassid S, Salmon I, et al. Differential expression of S100 calcium-binding proteins in epidermoid cysts, branchial cysts, craniopharyngiomas and cholesteatomas. Histopathology. 2003; 4. 42(4):387–394. PMID: 12653951.
Article14. Knudsen LM, Pallesen G. The preservation and loss of various non-haematopoietic antigens in human post-mortem tissues as demonstrated by monoclonal antibody immunohistological staining. Histopathology. 1986; 10. 10(10):1007–1014. PMID: 3096867.
Article15. Rorive S, Eddafali B, Fernandez S, Decaestecker C, Andre S, Kaltner H, et al. Changes in galectin-7 and cytokeratin-19 expression during the progression of malignancy in thyroid tumors: diagnostic and biological implications. Mod Pathol. 2002; 12. 15(12):1294–1301. PMID: 12481010.
Article16. Mathieu A, Saal I, Vuckovic A, Ransy V, Vereerstraten P, Kaltner H, et al. Nuclear galectin-3 expression is an independent predictive factor of recurrence for adenocarcinoma and squamous cell carcinoma of the lung. Mod Pathol. 2005; 9. 18(9):1264–1271. PMID: 15832191.
Article17. D'Haene N, Maris C, Sandras F, Dehou MF, Remmelink M, Decaestecker C, et al. The differential expression of galectin-1 and galectin-3 in normal lymphoid tissue and non-Hodgkin's and Hodgkin's lymphomas. Int J Immunopathol Pharmacol. 2005; Jul-Sep. 18(3):431–443. PMID: 16164826.18. Kojima H, Miyazaki H, Shiwa M, Tanaka Y, Moriyama H. Molecular biological diagnosis of congenital and acquired cholesteatoma on the basis of differences in telomere length. Laryngoscope. 2001; 5. 111(5):867–873. PMID: 11359168.
Article19. Olszewska E, Lautermann J, Koc C, Schwaab M, Dazert S, Hildmann H, et al. Cytokeratin expression pattern in congenital and acquired pediatric cholesteatoma. Eur Arch Otorhinolaryngol. 2005; 9. 262(9):731–736. PMID: 15754169.
Article20. Sarafian V, Jans R, Poumay Y. Expression of lysosome-associated membrane protein 1 (Lamp-1) and galectins in human keratinocytes is regulated by differentiation. Arch Dermatol Res. 2006; 7. 298(2):73–81. PMID: 16710742.
Article21. Dabbs DJ. Diagnostic immunohistochemistry. 2002. New York: Churchill Livingstone.22. Saussez S, Decaestecker C, Lorfevre F, Chevalier D, Mortuaire G, Kaltner H, et al. Increased expression and altered intracellular distribution of adhesion/growth-regulatory lectins galectins-1 and -7 during tumour progression in hypopharyngeal and laryngeal squamous cell carcinomas. Histopathology. 2008; 3. 52(4):483–493. PMID: 18315601.
Article23. Akimoto Y, Hirabayashi J, Kasai K, Hirano H. Expression of the endogenous 14-kDa beta-galactoside-binding lectin galectin in normal human skin. Cell Tissue Res. 1995; 4. 280(1):1–10. PMID: 7750127.24. Lacina L, Plzakova Z, Smetana K Jr, Stork J, Kaltner H, Andre S. Glycophenotype of psoriatic skin. Folia Biol (Praha). 2006; 52(1-2):10–15. PMID: 17007105.25. Simon P, Decaestecker C, Choufani G, Delbrouck C, Danguy A, Salmon I, et al. The levels of retinoid RARbeta receptors correlate with galectin-1, -3 and -8 expression in human cholesteatomas. Hear Res. 2001; 6. 156(1-2):1–9. PMID: 11377877.26. Haake AR, Cooklis M. Incomplete differentiation of fetal keratinocytes in the skin equivalent leads to the default pathway of apoptosis. Exp Cell Res. 1997; 2. 231(1):83–95. PMID: 9056414.
Article27. Saegusa J, Hsu DK, Liu W, Kuwabara I, Kuwabara Y, Yu L, et al. Galectin-3 protects keratinocytes from UVB-induced apoptosis by enhancing AKT activation and suppressing ERK activation. J Invest Dermatol. 2008; 10. 128(10):2403–2411. PMID: 18463681.
Article28. Delorge S, Saussez S, Pelc P, Devroede B, Marchant H, Burchert M, et al. Correlation of galectin-3/galectin-3-binding sites with low differentiation status in head and neck squamous cell carcinomas. Otolaryngol Head Neck Surg. 2000; 6. 122(6):834–841. PMID: 10828795.
Article29. Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A model for p53-induced apoptosis. Nature. 1997; 9. 389(6648):300–305. PMID: 9305847.
Article30. Kuwabara I, Kuwabara Y, Yang RY, Schuler M, Green DR, Zuraw BL, et al. Galectin-7 (PIG1) exhibits pro-apoptotic function through JNK activation and mitochondrial cytochrome c release. J Biol Chem. 2002; 2. 277(5):3487–3497. PMID: 11706006.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Distribution of Cytokeratins in Cholesteatomas according to the Induction Period of Cholesteatoma in Gerbil
- Compound Nevus Occurring Near External Auditory Canal: Successful Treatment by CO2 Laser Abrasion
- A Case of Intradermal Nevus of the External Auditory Canal
- Two Cases of Intradermal Nevus of the External Auditory Canal
- Acquired Atresia of External Auditory Canal Associated With Synovial Chondromatosis of the Temporomandibular Joint