J Korean Ophthalmol Soc.
2009 Mar;50(3):440-449. 10.3341/jkos.2009.50.3.440.
Histopathological Changes by Low-Power-Long-Duration and High-Power-Short-Duration Subthreshold Laser Treatment in the Rabbit Retina
- Affiliations
-
- 1Department of Ophthalmology, Maryknoll Hospital, Pusan, Korea. jooeun2@korea.com
- 2Department of Ophthalmology, School of Medicine, Pusan National University, Pusan, Korea.
- KMID: 2111257
- DOI: http://doi.org/10.3341/jkos.2009.50.3.440
Abstract
-
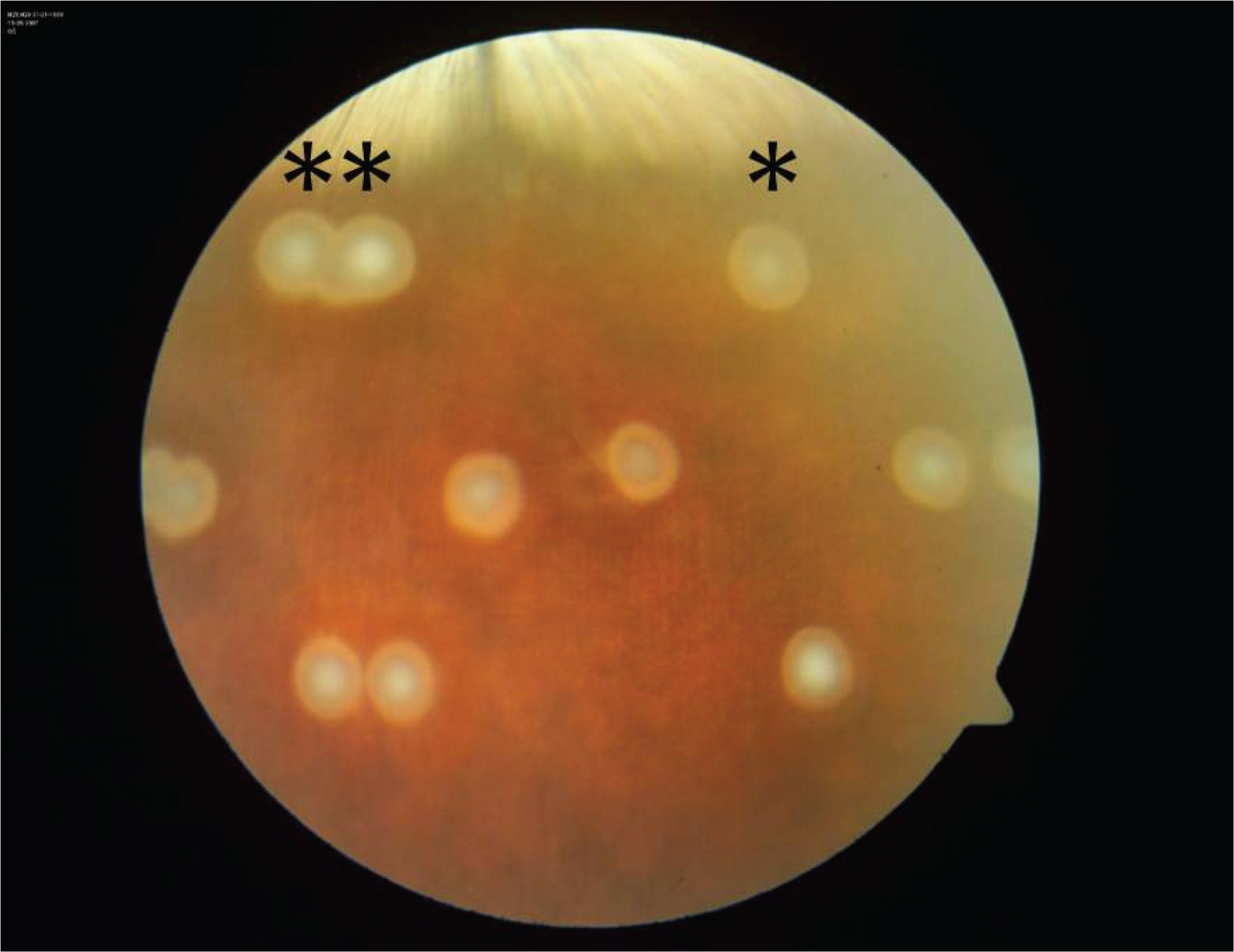
PURPOSE: To compare histopathological and apoptotic changes of ophthalmoscopically similar subthreshold laser burns made by a low power-long duration (LD) and a high power-short duration (SD) subthreshold laser treatment.
METHODS
Ophthalmoscopically invisible subthreshold laser burns with a 3.0 mm spot size were made using an 810 nm diode laser on the rabbit retina. Lasers were applied for 60 seconds in the LD group, and 1 second in the SD group. Laser power was adjusted to achieve ophthalmoscopically invisible burns just below the threshold. The rabbits were sacrificed at 6, 12, 24, and 72 hours, 1, 2, and 4 weeks after laser treatment. The eyes were processed for light microscopic examination using hematoxylin and eosin (H&E), toluidine blue, and TdT-dUTP terminal nick-end labeling (TUNEL) staining. Eyes were also processed for electron microscopic examination.
RESULTS
The changes in the retina were different between the two groups. The LD group showed abundant TUNEL positive cells in all the retinal layers at 6 hours after laser treatment, and distinct histological changes in the outer nuclear layer. Conversely, in the SD group, apoptosis did not occur and histological alteration in the outer nuclear layer was minimal.
CONCLUSIONS
Subthreshold laser treatment for 1 second reduced damage of the inner retinal layer and did not result in apoptosis in the neurosensory retina while maintaining a similar effect on the RPE and its adjacent region.
MeSH Terms
Figure
Reference
-
References
1. Macular Photocoagulation Study Group. Laser photocoagulation of subfoveal neovascular lesions in age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991; 109:1220–31.2. Macular Photocoagulation Study Group. Subfoveal neovascular lesions in age-related macular degeneration: results of a randomized clinical trial. Arch Ophthalmol. 1991; 109:1232–41.3. Oosterhuis JA, Journee-de Korver HG, Kakebeeke-Kemme HM, Bleeker JC. Transpupillary thermotherapy in choroidal melanomas. Arch Ophthalmol. 1995; 113:315–21.
Article4. Shields CL, Shields JA, Cater J, et al. Transpupillary thermotherapy for choroidal melanoma: tumor control and visual results in 100 consecutive cases. Ophthalmology. 1998; 105:581–90.5. Kim US, Yu SY, Kim SW, Kwak HW. Five cases of transpupillary thermotherapy for intraocular tumors. J Korean Ophthalmol Soc. 2003; 44:2942–49.6. Oh JW, Yun IH. Transpupillary thermotherapy in circumscribed choroidal hemangioma. J Korean Ophthalmol Soc. 2003; 44:992–7.7. Reichel E, Berrocal AM, Ip M, et al. Transpupillary thermotherapy of occult subfoveal choroidal neovascularization in patients with age-related macular degeneration. Ophthalmology. 1999; 106:1908–14.
Article8. Newsom RS, McAlister JC, Saeed M, McHugh JD. Transpupillary thermotherapy (TTT) for the treatment of choroidal neovascularization. Br J Ophthalmol. 2001; 85:173–8.9. Algvere PV, Libert C, Seregard S. Transpupillary thermotherapy of occult CNV with no or minimally classic CNV in age-related macular degeneration. Semin Ophthalmol. 2001; 16:90–6.
Article10. Morimura Y, Okada AA, Hayashi A, et al. Histological effect and protein expression in subthreshold transpupillary thermotherapy in rabbit eyes. Arch Ophthalmol. 2004; 122:1510–5.11. She H, Li X, Yu W. Subthreshold transpupillary thermotherapy of the retina and experimental choroidal neovascularization in a rat model. Graefes Arch Clin Exp Ophthalmol. 2006; 244:1143–51.
Article12. Welch AJ, Wissler EH, Priebe LA. Significance of blood flow in calculation of temperature in laser irradiated tissue. IEEE Trans Biomed Eng. 1980; 27:164–6.13. Welch AJ, Polhamus GD. Measurement and prediction of thermal injury in the retina of the rhesus monkey. IEEE Trans Biomed Eng. 1984; 31:633–43.
Article14. Parver LM, Auker C, Carpenter DO. Choroidal blood flow as a heat dissipating mechanism in the macula. Am J Ophthalmol. 1980; 89:641–6.
Article15. Parver LM. Temperature modulating action of choroidal blood flow. Eye. 1991; 5:181–5.
Article16. Mainster MA, White TJ, Tips JH, Wilson PW. Retinal- temperature increases produced by intense light sources. J Opt Soc Am. 1970; 60:264–70.17. Vassiliadis A. Laser sources and ocular effects. Francis A, L'esperance JR, editors. Ophthalmic lasers. 3rd ed.St. Louis: The C.V. Mosby company;1989. 1:chap. 2.18. Mainster MA. Decreasing retinal photocoagulation damage: principles and techniques. Semin Ophthalmol. 1999; 14:200–9.
Article19. Desmettre T, Maurage CA, Mordon S. Heat shock protein hyperexpression on chorioretinal layers after transpupillary thermot-herapy. Invest Ophthalmol Vis Sci. 2001; 42:2976–80.20. Desmettre T, Maurage CA, Mordon S. Transpupillary thermotherapy (TTT) with short duration laser exposure induce heat shock protein (HSP) hyperexpression on choroidoretinal layers. Lasers Surg Med. 2003; 33:102–7.21. Mainster MA, Reichel E. Transpupillary thermotherapy for age-related macular degeneration: long-pulse photocoagulation, apoptosis, and heat shock proteins. Ophthalmic Surg Lasers. 2000; 31:359–73.
Article22. Samali A, Orrenius S. Heat shock proteins: regulators of stress response and apoptosis. Cell Stress Chaperones. 1998; 3:228–36.
Article23. Mainster MA. Wavelength selection in macular photocoagulation. Tissue optics, thermal effects, and laser systems. Ophthalmology. 1986; 93:952–8.24. Berger JW. Thermal modelling of micropulsed diode laser retinal photocoagulation. Lasers Surg Med. 1997; 20:409–15.
Article25. Ming Y, Alqvere PV, Oderqren A, et al. Subthreshold transpupillary thermotherapy reduces experimental choroidal neovascularization in the mouse without collateral damage to the neural retina. Invest Ophthalmol Vis Sci. 2004; 45:1969–74.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Whole Layer Photocoagulation on the Rabbit Retina with Indirect Diode Laser Ophthalmoscopy
- An Experimental Study on the Bladder Tissue Damage with Neodymium-YAG Laser Irradiation in Rabbit
- Histopathologic and ultrastructural findings of photocoagulation lesions produced by transpupillary diode laser in the rabbit retina
- Changes in adhesive force between the retina and the retinal pigment epithelium by laser photocoagulation in rabbits
- Comparison of Photocoagulation with the Argon and Diode Laser in Rabbit Eyes