J Korean Soc Magn Reson Med.
2012 Dec;16(3):205-216. 10.13104/jksmrm.2012.16.3.205.
Invasive Micropapillary Carcinoma of the Breast: Mammographic, Sonographic and MR Imaging Findings
- Affiliations
-
- 1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. bkhan@skku.edu
- KMID: 2099856
- DOI: http://doi.org/10.13104/jksmrm.2012.16.3.205
Abstract
- PURPOSE
We performed this study to investigate the characteristic imaging and clinicopathologic features of invasive micropapillary carcinoma of the breast.
MATERIALS AND METHODS
Among the 47 women with surgically confirmed invasive micropapillary carcinoma between 2005 and 2009, 32 patients (mean age, 50 years; range, 37-69 years) had all preoperative mammography, ultrasound (US) and MR images. Two radiologists retrospectively assessed the imaging findings, clinical presentation and histological results of the patients.
RESULTS
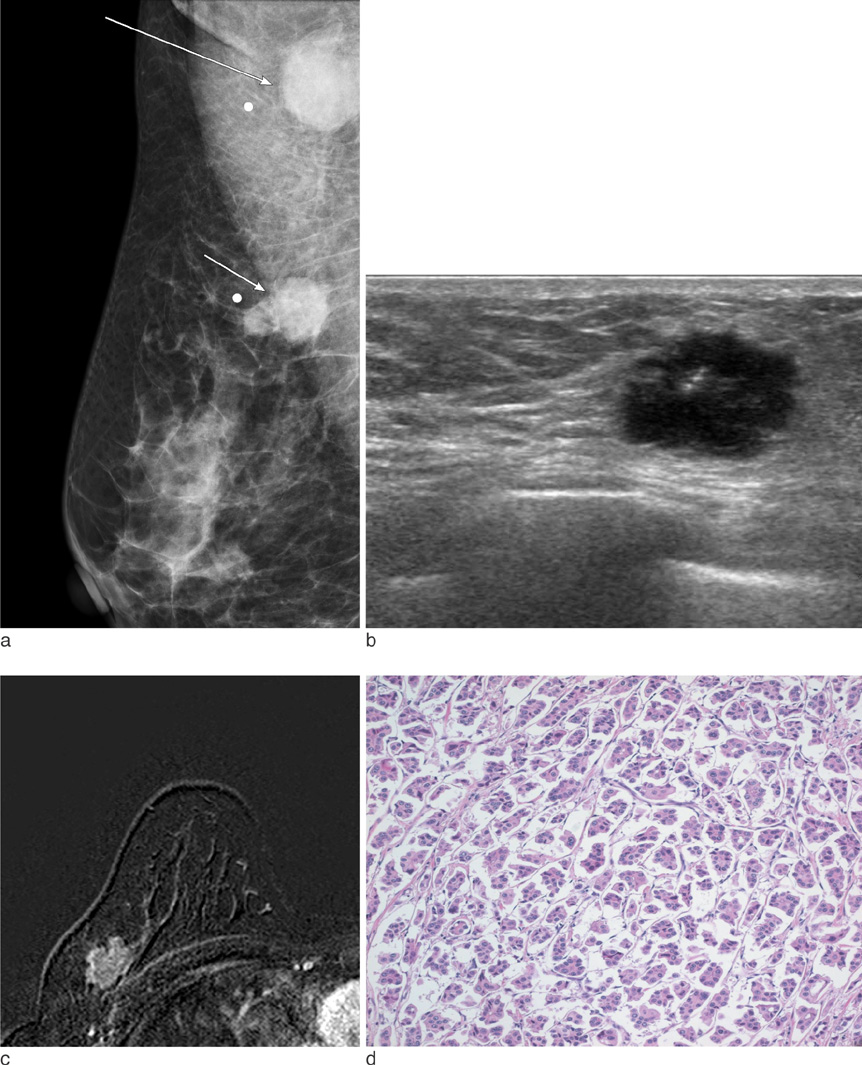
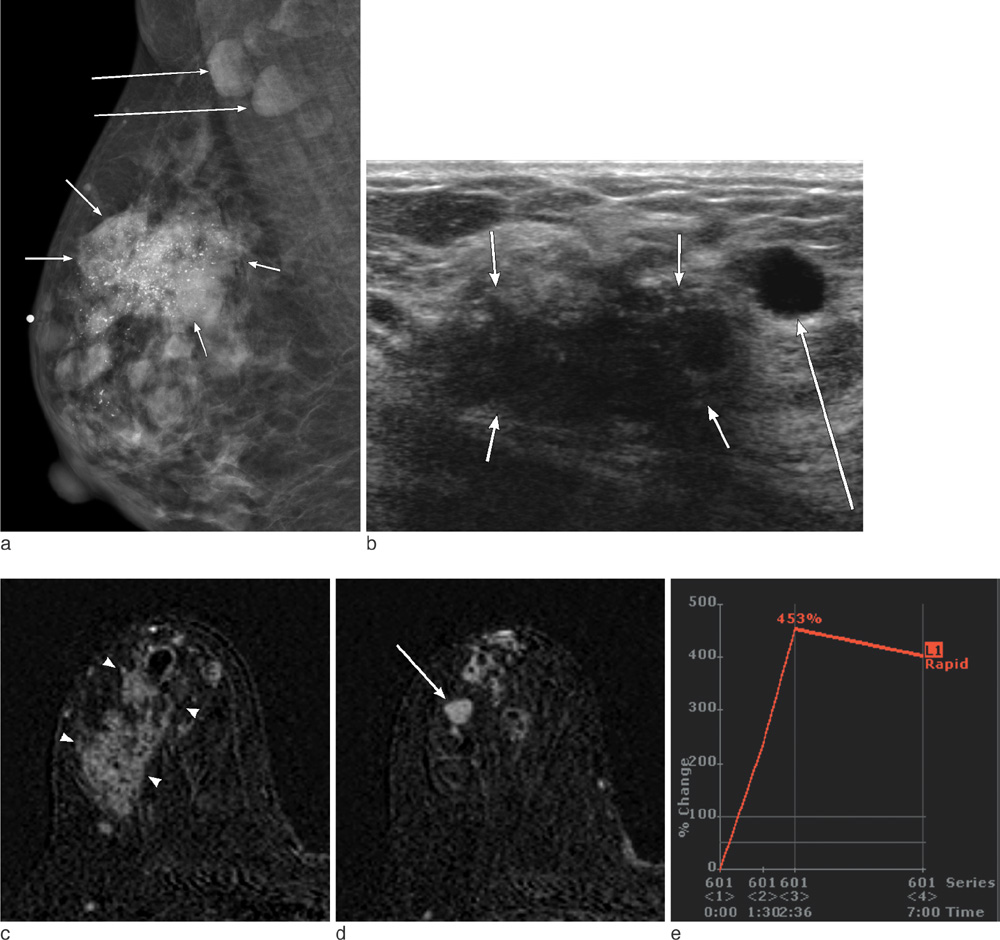
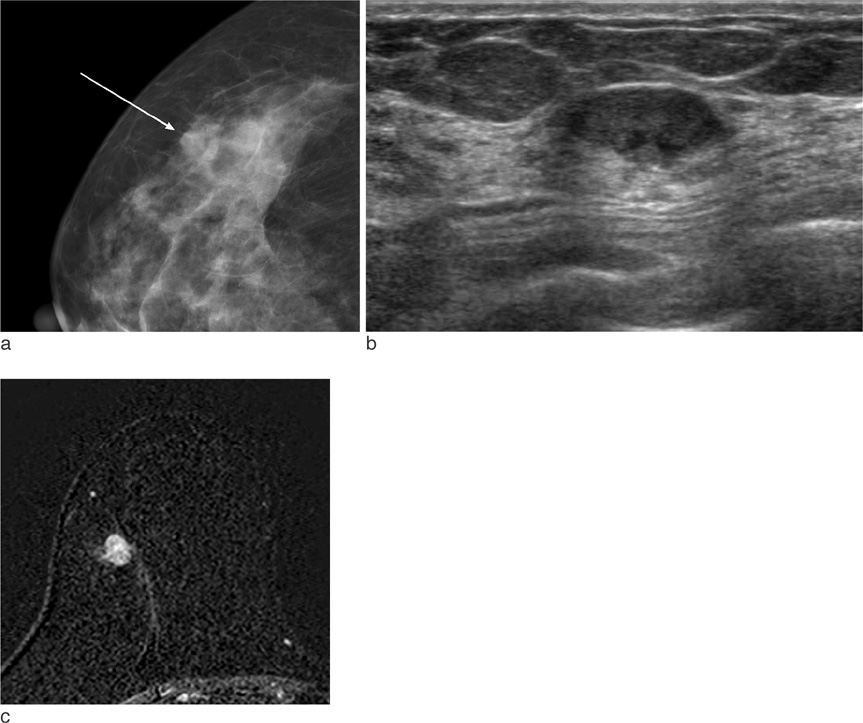
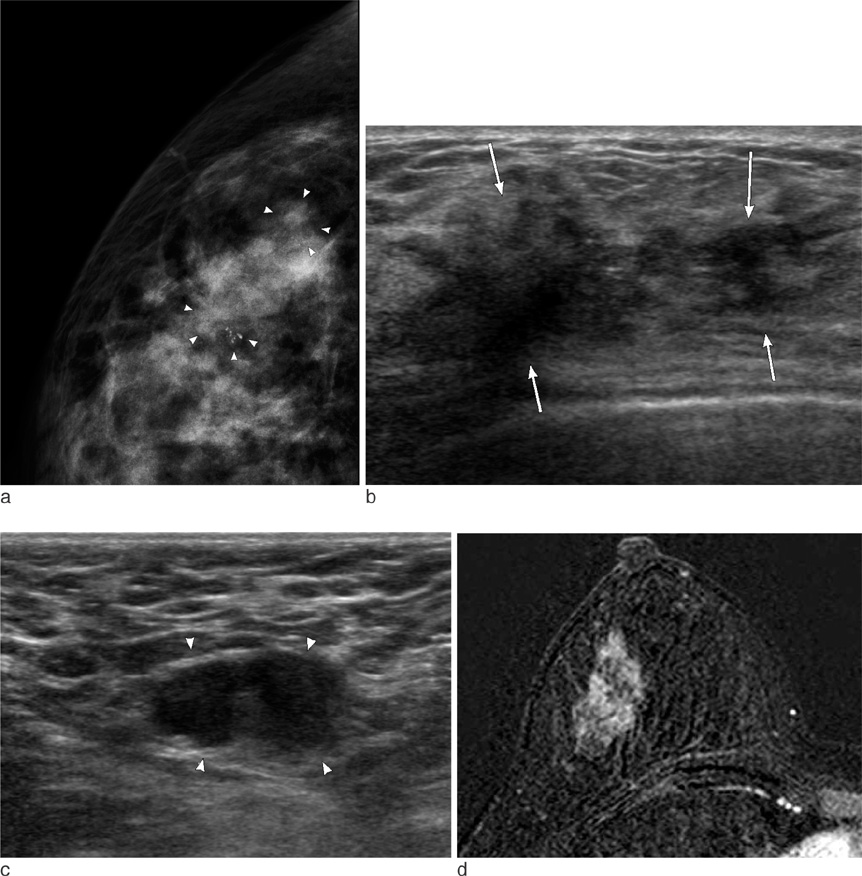
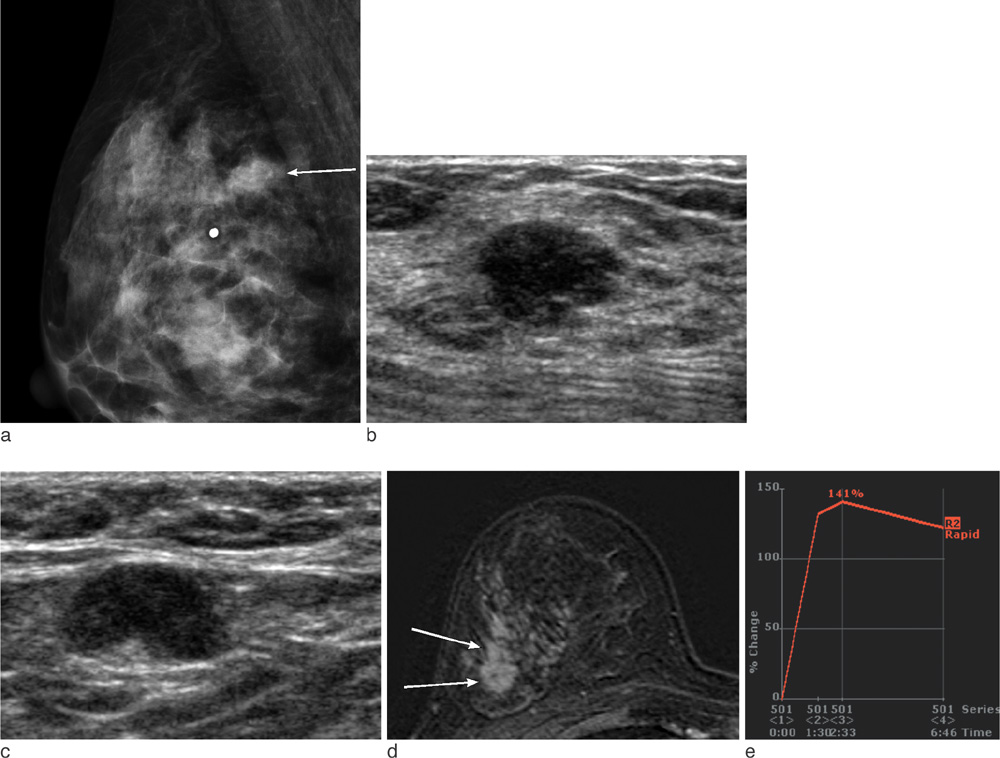
On mammography, 29 of 32 patients had suspicious findings. Among them, a mass (or focal asymmetry) with calcifications was the most common findings (15/32, 65%). The calcifications were noted in 20 patients (63%) and the shape of calcifications was frequently amorphous or punctate (n = 12, 60%). On US and MR imaging, all lesions had suspicious findings. The most common US findings were single (n = 20) or multiple (n = 10) irregular hypoechoic mass (es). The mass was frequently hypoechoic (n = 29, 97%). On MR imaging, the type of lesions was a mass or masses in 23 (72%), a mass combined with non-mass in six patients, and non-mass lesions in three patients. Histologically, axillary lymph nodes metastasis were very common (25/32, 78%). Asymptomatic clinical presentation was not usual (9/32, 28%).
CONCLUSION
The imaging features of invasive micropapillary carcinomas strongly suggest malignancy. Microcalcifications on mammography, marked hypoechogenicity on US and an irregular mass, often combined with non-mass on MR are common. Axillary lymph node metastasis is commonly associated.
MeSH Terms
Figure
Reference
-
1. Siriaunkgul S, Tavassoli FA. Invasive micropapillary carcinoma of the breast. Mod Pathol. 1993. 6:660–662.2. Petersen JL. Breast carcinomas with an unexpected inside-out growth pattern: rotation of polarization associated with angioinvasion (abstr). Pathol Res Pract. 1993. 189:780–784.3. Luna-Moré S, Gonzalez B, Acedo C, Rodrigo I, Luna C. Invasive micropapillary carcinoma of the breast. A new special type of invasive mammary carcinoma. Pathol Res Pract. 1994. 190:668–667.4. Middleton LP, Tressera F, Sobel ME, et al. Infiltrating micropapillary carcinoma of the breast. Mod Pathol. 1999. 12:499–504.5. Nassar H, Wallis T, Andea A, Dey J, Adsay V, Visscher D. Clinicopathologic analysis of invasive micropapillary differentiation in breast carcinoma. Mod Pathol. 2001. 14:836–841.6. Paterakos M, Watkin WG, Edgerton SM, Moore DH 2nd, Thor AD. Invasive micropapillary carcinoma of the breast: a prognostic study. Hum Pathol. 1999. 30:1459–1463.7. Adrada B, Arribas E, Gilcrease M, Yang WT. Invasive micropapillary carcinoma of the breast: mammographic, sonographic, and MRI features. AJR Am J Roentgenol. 2009. 193:W58–W63.8. Günhan-Bilgen I, Zekioglu O, Ustün EE, Memis A, Erhan Y. Invasive micropapillary carcinoma of the breast: clinical, mammographic, and sonographic findings with histopathologic correlation. AJR Am J Roentgenol. 2002. 179:927–931.9. Radiology, A.C.o.Mammography, in Breast imaging reporting and data system (BI-RADS). 2003. Reston: American College of Radiology.10. Soo MS, Williford ME, Walsh R, Bentley RC, Kornguth PJ. Papillary carcinoma of the breast: imaging findings. AJR Am J Roentgenol. 1995. 164:321–326.11. Muttarak M, Lerttumnongtum P, Chaiwun B, Peh WC. Spectrum of papillary lesions of the breast: clinical, imaging, and pathologic correlation. AJR Am J Roentgenol. 2008. 191:700–707.12. Ibarra JA. Papillary lesions of the breast. Breast J. 2006. 12:237–251.13. Kim DS, Nariya C, Ko ES, et al. Imaging and the clinical-pathologic features of invasive micropapillary carcinoma of the breast. J Korean Radiol Soc. 2007. 56:497–503.14. Chiou HJ, Chou YH, Chiou SY, et al. Superficial soft-tissue lymphoma: sonographic appearance and early survival. Ultrasound Med Biol. 2006. 32:1287–1297.15. Daniel BL, Yen YF, Glover GH, et al. Breast disease: dynamic spiral MR imaging. Radiology. 1998. 209:499–509.16. Snow RD, Dyess DL, Harpen MD, Kreisberg CN, Tucker JA. Dynamic magnetic resonance imaging in evaluating suspicious breast lesions: correlation with pathologic findings. South Med J. 1998. 91:527–532.17. Pettinato G, Manivel CJ, Panico L, Sparano L, Petrella G. Invasive micropapillary carcinoma of the breast: clinicopathologic study of 62 cases of a poorly recognized variant with highly aggressive behavior. Am J Clin Pathol. 2004. 121:857–866.18. Zekioglu O, Erhan Y, Ciris M, Bayramoglu H, Ozdemir N. Invasive micropapillary carcinoma of the breast: high incidence of lymph node metastasis with extranodal extension and its immunohistochemical profile compared with invasive ductal carcinoma. Histopathology. 2004. 44:18–23.19. Luna-Moré S, de los Santos F, Bretón JJ, Cañadas MA. Estrogen and progesterone receptors, c-erbB-2, p53, and Bcl-2 in thirty-three invasive micropapillary breast carcinomas. Pathol Res Pract. 1996. 192:27–32.20. Walsh MM, Bleiweiss IJ. Invasive micropapillary carcinoma of the breast: eighty cases of an underrecognized entity. Hum Pathol. 2001. 32:583–589.21. Yun SU, Choi BB, Shu KS, et al. Imaging findings of invasive micropapillary carcinoma of the breast. J Breast Cancer. 2012. 15:57–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Imaging and the Clinical-Pathologic Features of Invasive Micropapillary Carcinoma of the Breast
- Invasive Micropapillary Carcinoma in Breast Presented as Hyperechoic Mass with Coarse Macrocalcifications: A Case Report
- Invasive Micropapillary Carcinoma of the Breast: A clinicopathologic study of 16 cases
- Invasive Micropapillary Carcinoma of the Breast: MR Imaging Findings
- Imaging Findings of Invasive Micropapillary Carcinoma of the Breast