Korean J Hematol.
2010 Jun;45(2):115-119. 10.5045/kjh.2010.45.2.115.
Soluble syndecan-1 at diagnosis and during follow up of multiple myeloma: a single institution study
- Affiliations
-
- 1Department of Laboratory Medicine, Eulji University Hospital, Daejeon, Korea. jmkim@eulji.ac.kr
- 2Department of Haemato-Oncology, Eulji University Hospital, Daejeon, Korea.
- KMID: 2083544
- DOI: http://doi.org/10.5045/kjh.2010.45.2.115
Abstract
- BACKGROUND
Syndecan-1 is a heparan sulfate proteoglycan expressed on plasma cells, especially myeloma cells, and can exist in serum as soluble syndecan-1 after shedding from the cell surface. Soluble syndecan-1 has been suggested to promote myeloma cell growth and to be an independent prognostic factor for multiple myeloma. We aimed to evaluate the effect of soluble syndecan-1 levels at the time of diagnosis and during therapy on therapeutic response and prognosis for patients with multiple myeloma.
METHODS
We analyzed soluble syndecan-1 levels in 28 patients with multiple myeloma and 50 normal controls, and compared its levels with Durie-Salmon stage and other markers of myeloma. In addition, we evaluated the therapeutic response and determined the 3-year survival rates of these patients.
RESULTS
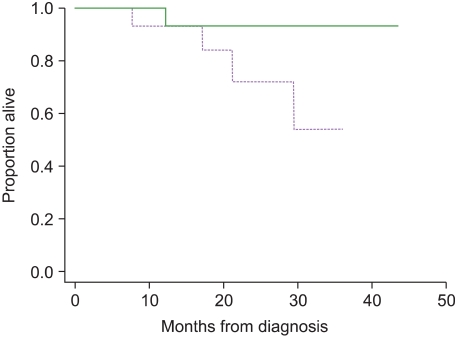
We observed that the median soluble syndecan-1 level in myeloma patients was higher than that in the normal controls (P <0.0001), and the soluble syndecan-1 levels in 21 (75%) patients were higher than the cut-off level (162 ng/mL). Soluble syndecan-1 levels correlated with disease stage, percentage of plasma cells in the bone marrow, beta2 microglobulin level, serum M-component concentration, and creatinine level. The baseline levels of soluble syndecan-1 at the time of diagnosis in the patients who responded to chemotherapy were lower than those in the non-responders (P=0.04); however, the baseline level was not a significant predictor of therapeutic response. The 3-year overall survival rate of the patients with high soluble syndecan-1 levels at the time of diagnosis and 6 months after chemotherapy was lower than the corresponding survival rates of the patients with low levels of soluble syndecan-1; however, the overall survival rate was not statistically significant.
CONCLUSION
The use of soluble syndecan-1 has limitations in the diagnosis of multiple myeloma. Soluble syndecan-1 levels correlate with known prognostic factors; however, we could not assess the prognostic value of high levels of soluble syndecan-1 at the time of diagnosis and after chemotherapy.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Soluble syndecan-1 (CD138): is it useful as a prognostic factor in Korean patients with multiple myeloma?
Je-Jung Lee
Korean J Hematol. 2010;45(3):143-144. doi: 10.5045/kjh.2010.45.3.143.
Reference
-
1. Costes V, Magen V, Legouffe E, et al. The Mi15 monoclonal antibody (anti-syndecan-1) is a reliable marker for quantifying plasma cells in paraffin-embedded bone marrow biopsy specimens. Hum Pathol. 1999; 30:1405–1411. PMID: 10667416.
Article2. Sanderson RD, Børset M. Syndecan-1 in B lymphoid malignancies. Ann Hematol. 2002; 81:125–135. PMID: 11904737.
Article3. Langford JK, Yang Y, Kieber-Emmons T, Sanderson RD. Identification of an invasion regulatory domain within the core protein of syndecan-1. J Biol Chem. 2005; 280:3467–3473. PMID: 15563454.
Article4. Dhodapkar MV, Kelly T, Theus A, Athota AB, Barlogie B, Sanderson RD. Elevated levels of shed syndecan-1 correlate with tumour mass and decreased matrix metalloproteinase-9 activity in the serum of patients with multiple myeloma. Br J Haematol. 1997; 99:368–371. PMID: 9375756.
Article5. Seidel C, Sundan A, Hjorth M, et al. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood. 2000; 95:388–392. PMID: 10627439.
Article6. Lovell R, Dunn JA, Begum G, et al. Soluble syndecan-1 level at diagnosis is an independent prognostic factor in multiple myeloma and the extent of fall from diagnosis to plateau predicts for overall survival. Br J Haematol. 2005; 130:542–548. PMID: 16098068.
Article7. Durie BG, Salmon SE. A clinical staging system for multiple myeloma: correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer. 1975; 36:842–854. PMID: 1182674.
Article8. Smith A, Wisloff F, Samson D. UK myeloma Forum, Nordic Myeloma Study Group, British Committee for Standards in Haematology. Guidelines on the diagnosis and management of multiple myeloma 2005. Br J Haematol. 2006; 132:410–451. PMID: 16412016.
Article9. Yang Y, Yaccoby S, Liu W, et al. Soluble syndecan-1 promotes growth of myeloma tumors in vivo. Blood. 2002; 100:610–617. PMID: 12091355.10. Kumar S, Blood E, Oken MM, Greipp PR. Prognostic value of syndecan-1 in multiple myeloma and its relationship with other prognostic factors. Blood. 2004; 104(11):2402.
Article11. Jánosi J, Sebestyén A, Mikala G, Németh J, Kiss Z, Vályi-Nagy I. Soluble syndecan-1 levels in different plasma cell dyscrasias and in different stages of multiple myeloma. Haematologica. 2004; 89:370–371. PMID: 15020284.12. Kyrtsonis MC, Vassilakopoulos TP, Siakantaris MP, et al. Serum syndecan-1, basic fibroblast growth factor and osteoprotegerin in myeloma patients at diagnosis and during the course of the disease. Eur J Haematol. 2004; 72:252–258. PMID: 15089762.
Article13. Schaar CG, Vermeer HJ, Wijermans PW, Huisman W, le Cessie S, Kluin-Nelemans HC. Serum syndecan-1 in patients with newly diagnosed monoclonal proteinemia. Haematologica. 2005; 90:1437–1438. PMID: 16219583.14. Fassas AB, Spencer T, Sawyer J, et al. Both hypodiploidy and deletion of chromosome 13 independently confer poor prognosis in multiple myeloma. Br J Haematol. 2002; 118:1041–1047. PMID: 12199783.
Article15. Rajkumar SV, Kyle RA, Therneau TM, et al. Serum free light chain ratio is an independent risk factor for progression in monoclonal gammopathy of undetermined significance. Blood. 2005; 106:812–817. PMID: 15855274.
Article16. Joshua DE, Brown RD, Gibson J. Prognostic factors in myeloma: what they tell us about the pathophysiology of the disease. Leuk Lymphoma. 1994; 15:375–381. PMID: 7873994.
Article17. Salmon SE, Smith BA. Immunoglobulin synthesis and total body tumor cell number in IgG multiple myeloma. J Clin Invest. 1970; 49:1114–1121. PMID: 4987170.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Soluble syndecan-1 (CD138): is it useful as a prognostic factor in Korean patients with multiple myeloma?
- A Case of Acute Promyelocytic Leukemia Concomitant with Plasma Cell Myeloma
- Clinical Application of (18)F-FDG PET in Multiple Myeloma
- Syndecan-1 (sCD138) levels in chronic lymphocytic leukemia: clinical and hematological correlations
- Multiple myeloma