J Vet Sci.
2014 Dec;15(4):579-582. 10.4142/jvs.2014.15.4.579.
Eggshell apex abnormalities associated with Mycoplasma synoviae infection in layers
- Affiliations
-
- 1Avian Disease Laboratory, College of Veterinary Medicine, Chungbuk National University, Cheongju 361-763, Korea. moip@cbu.ac.kr
- 2Aviagen, Huntsville, AL 35805, USA.
- KMID: 2070247
- DOI: http://doi.org/10.4142/jvs.2014.15.4.579
Abstract
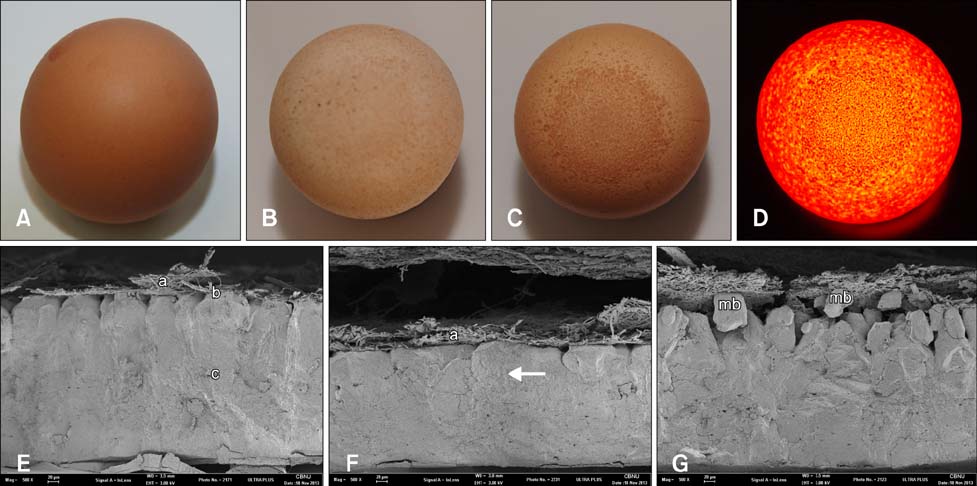
- Eggs exhibiting eggshell apex abnormalities (EAA) were evaluated for changes in shell characteristics such as strength, thickness, and ultrastructure. Mycoplasma synoviae (MS) infection was confirmed by serological assay along with isolation of MS from the trachea and oviduct. Changes in eggshell quality were shown to be statistically significant (p < 0.01). We also identified ultrastructural changes in the mammillary knob layer by Scanning Electron Microscopy. While eggs may seem to be structurally sound, ultrastructural evaluation showed that affected eggs do not regain their former quality. In our knowledge, this is the first report describing the occurrence of EAA in Korea.
Keyword
MeSH Terms
Figure
Reference
-
1. Bunk MJ, Balloun SL. Ultrastructure of the mammillary region of low puncture strength avian eggshells. Poult Sci. 1978; 57:639–647.
Article2. Catania S, Bilato D, Gobbo F, Granato A, Terregino C, Iob L, Nicholas RA. Treatment of eggshell abnormalities and reduced egg production caused by Mycoplasma synoviae infection. Avian Dis. 2010; 54:961–964.
Article3. Dufour-Gesbert F, Dheilly A, Marois C, Kempf I. Epidemiological study on Mycoplasma synoviae infection in layers. Vet Microbiol. 2006; 114:148–154.4. Feberwee A, de Wit JJ, Landman WJM. Induction of eggshell apex abnormalities by Mycoplasma synoviae: field and experimental studies. Avian Pathol. 2009; 38:77–85.
Article5. Feberwee A, Morrow CJ, Ghorashi SA, Noormohammadi AH, Landman WJM. Effect of a live Mycoplasma synoviae vaccine on the production of eggshell apex abnormalities induced by a M. synoviae infection preceded by an infection with infectious bronchitis virus D1466. Avian Pathol. 2009; 38:333–340.
Article6. Gole VC, Chousalkar KK, Roberts JR. Prevalence of antibodies to Mycoplasma synoviae in laying hens and possible effects on egg shell quality. Prev Vet Med. 2012; 106:75–78.
Article7. Jeffery N, Gasser RB, Steer PA, Noormohammadi AH. Classification of Mycoplasma synoviae strains using single-strand conformation polymorphism and highresolution melting-curve analysis of the vlhA gene single-copy region. Microbiology. 2007; 153:2679–2688.
Article8. Jordan FTW. Avian mycoplasma and pathogenicity - a review. Avian Pathol. 1975; 4:165–174.
Article9. Lee HR, Kim JM, Kim JH, Kim CM, So HH, Lee DW, Ha BD, Hong SC, Mo IP. Serological survey for the major viral diseases in the layers. Korea J Poult Sci. 2010; 37:361–372.
Article10. Lysnyansky I, Gerchman I, Mikula I, Gobbo F, Catania S, Levisohn S. Molecular characterization of acquired enrofloxacin resistance in Mycoplasma synoviae field isolates. Antimicrob Agents Chemother. 2013; 57:3072–3077.
Article11. MacOwan KJ, Randall CJ, Jones HGR, Brand TF. Association of Mycoplasma synoviae with respiratory disease of broilers. Avian Pathol. 1982; 11:235–244.12. Mohammed HO, Carpenter TE, Yamamoto R. Economic impact of Mycoplasma gallisepticum and M. synoviae in commercial layer flocks. Avian Dis. 1987; 31:477–482.
Article13. Roberts JR. Factors affecting egg internal quality and egg shell quality in laying hens. J Poult Sci. 2004; 41:161–177.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development and evaluation of semi-nested PCR for detection of the variable lipoprotein haemagglutinin (vlhA) gene of Mycoplasma Synoviae in chicken
- Epidemiology of Mycoplasma pneumoniae Infection in Childhood
- Clinical Study of Mycoplasma Pneumoniae Infection in Children
- Respiratory infection by mycoplasma pneumoniae
- Epidemiology and diagnosis of Mycoplasma pneumoniae infection