J Korean Diabetes Assoc.
2006 Mar;30(2):96-103. 10.4093/jkda.2006.30.2.96.
Effects of Pioglitazone on Cerebral Hemodynamics in Patients of Type 2 Diabetes
- Affiliations
-
- 1Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea.
- 2Ewha Graduate School of Clinical Health Science, Seoul, Korea.
- 3Department of Neurology, Yonsei University College of Medicine, Seoul, Korea.
- 4Department of Internal Medicine, Kwandong University College of Medicine, Gangneung, Korea.
- KMID: 2063438
- DOI: http://doi.org/10.4093/jkda.2006.30.2.96
Abstract
- BACKGROUND
Atherosclerosis is one of the major causes of morbidity and mortality in patients with type 2 diabetes and pioglitazone has been reported to have antiatherogenic effect. The aim of this study was to investigate whether pioglitazone affects carotid intima-media thickness (IMT) and pulsatility index (PI) in type 2 diabetic patients.
METHODS
A total of 40 type 2 diabetic patients were included and divided into two groups: the pioglitazone-treated group (pioglitazone 15 mg/day with gliclazide 80~320 mg/day for 12 weeks) (n = 20) and control group (gliclazide 80~320 mg/day for 12 weeks) (n = 20). The changes in lipid profile, insulin resistance, IMT, and PI were monitored to determine that pioglitazone improves cerebrovascular blood flow.
RESULTS
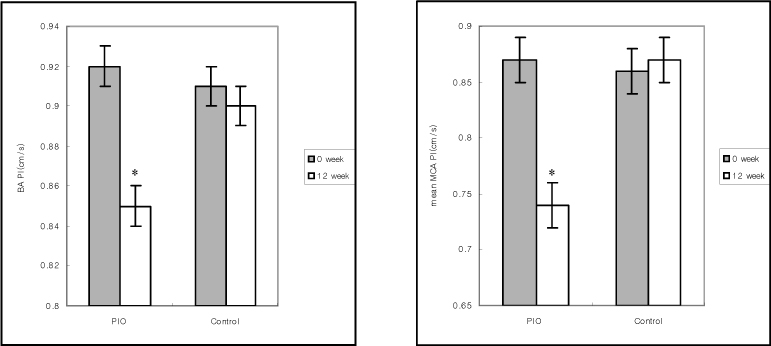
The pioglitazone treatment significantly increased HDL-C, reduced triglyceride, insulin resistance and PI. IMT tended to decrease but the change was not significant. This study revealed that treatment with pioglitazone was associated with the improvement of cerebrovascular blood flow.
CONCLUSIONS
Pioglitazone appears to be effective for the improvement of cerebrovascular blood flow in type 2 diabetic patients
MeSH Terms
Figure
Reference
-
1. Kannel WB, McGee DL. Diabetes and cardiovascular disease;the Framingham study. JAMA. 1979. 241:2035–2038.2. Yki-Jarvinen H. Thiazolidinediones. N Engl J Med. 2004. 351:1106–1118.3. Barbier O, Torra IP, Duguay Y, Blanquart C, Fruchart JC, Glineur C, Staels B. Pleiotropic actions of peroxisome proliferator-activated receptors in lipid metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol. 2002. 22:717–726.4. Martens FM, Visseren FL, Lemay J, De Koning EJ, Rabelink TJ. Metabolic and additional vascular effects of thiazolidinediones. Drugs. 2002. 62:1463–1480.5. Mercuri M, Bond MG, Nichos FT, Carr AA, Flack JM, Byington R, Raines J. Baseline reproducibility of B-mode ultrasound imaging measurements of carotid intimal media thickness. J Cardivasc Diagnosis Procedure. 1993. 11:241–252.6. Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors; the ARIC study, 1987-1993. Am J Epidemiol. 1997. 146:483–494.7. Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998. 128:262–269.8. O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK. Cardiovascular health study collaborative research group. Carotid artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. New Engl J Med. 1999. 340:14–22.9. Langenfeld MR, Forst T, Hohberg C, Konrad T, Fullert SD, Sachara C, Pfutzner A. Pioglitazone decrease carotid intima-media thickness independently of glycemic control in patients with type 2 diabetes mellitus. Circulation. 2005. 111:2525–2531.10. Nakamura T, Matsuda T, Kawagoe Y, Ogawa H, Takahashi Y, Sekizuka K, Koide H. Effect of pioglitazone on carotid intima-media thickness and arterial stiffness in type 2 diabetic nephropathy patients. Metabolism. 2004. 53:1382–1386.11. Meigs JB, Nathan DM, D'Agostino RB, Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002. 25:1845–1850.12. Amos AF, McCarty DJ, Zimmet P. The rising global burden of diabetes and its complications: estimates and projections to the year 2010. Diabet Med. 1997. 14:S7–S85.13. Wakisaka M, Nagamachi S, Inoue K, Morotomi Y, Nunoi K, Fujishima M. Reduced regional cerebral blood flow in aged noninsulin-dependent diabetic patients with no history of cerebrovascular disease: evaluation by N-isopropyl-123I-p-iodoamphetamine with single photon emission computed tomography. J Diabetes Complications. 1990. 4:170–174.14. Jimenez-Bonilla JF, Carril JM, Quirce R, Gomez-Barquin R, Amado JA, Gutierrez-Mendiguchia C. Assessment of cerebral blood flow in diabetic patients with no clinical history of neurological disease. Nucl Med Commun. 1996. 17:790–794.15. Rodriguez G, Nobili F, Celestino MA, Francione S, Gulli G, Hassan K, Marenco S, Rosadini G, Cordera R. Regional cerebral blood flow and cerebrovascular reactivity in IDDM. Diabetes Care. 1993. 16:462–483.16. Mortel KF, Meyer JS, Sims PA, McClintic K. Diabetes mellitus as a risk factor for stroke. South Med J. 1990. 83:904–911.17. Grill V, Gutniak M, Bjorkman O, Lindqvist M, Stone-Elander S, Seitz RJ, Blomqvist G, Reichard P, Widen L. Cerebral blood flow and substrate utilization in insulin-treated diabetic subjects. Am J Physiol. 1990. 258:E813–E820.18. Lee KY, Sohn YH, Baik JS, Kim GW, Kim JS. Arterial pulsatility as an index of cerebral microangiopathy in diabetes. Stroke. 2000. 31:1111–1115.20. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985. 28:412–419.21. Roberts AW, Thomas A, Rees A, Evans M. Peroxisome proliferators-activated receptor-r agonists in atherosclerosis: Current evidence and future directions. Curr Opin Lipidol. 2003. 14:567–573.22. Tham DM, Wang YX, Rutledge JC. Modulation of vascular inflammation by PPARs. Drug News Perspect. 2003. 16:109–116.23. Pftzner A, Marx N, Lbben G, Langenfeld M, Walcher D, Konrad T, Forst T. Improvement of cardiovascular risk markers by pioglitazone is independent from glycemic control: results from the pioneer study. J Am Coll Cardiol. 2005. 45:1925–1931.24. Derosa G, Cicero AF, Gaddi A, Ragonesi PD, Piccinni MN, Fogari E, Salvadeo S, Ciccarelli L, Fogari R. A comparison of the effects of pioglitazone and rosiglitazone combined with glimepiride on prothrombotic state in type 2 diabetic patients with the metabolic syndrome. Diabetes Res Clin Pract. 2005. 69:5–13.25. Boyle PJ, King AB, Olansky L, Marchetti A, Lau H, Martin J. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes melitus: a retrospective review of randomly selected medical records. Clin Ther. 2002. 24:378–396.26. Miyazaki Y, Mahankali A, Matsuda M, Glass L, Mahankali S, Ferrannini E, Cusi K, Mandarino LJ, DeFronzo RA. Improved glycemic control and enhanced insulin sensitivity in type 2 diabetic subjects treated with pioglitazone. Diabetes Care. 2001. 24:710–719.27. Yamanouchi T, Sakai T, Lgarashi K, Ichiyanagi K, Watanabe H, Kawasaki T. Comparison of metabolic effects of pioglitazone, metformin, and glimepiride over 1 year in Japanese patients with newly diagnosed Type 2 diabetes. Diabet Med. 2005. 22:980–985.28. Shimazu T, Inoue I, Araki N, Asano Y, Sawada M, Furuya D, Nagoya H, Greenberg JH. A peroxisome proliferator-activated receptor-gamma agonist reduces infarct size in transient but not in permanent ischemia. Stroke. 2005. 36:353–359.29. Sundararajan S, Gamboa JL, Victor NA, Wanderi EW, Lust WD, Landreth GE. Peroxisome proliferator-activated receptor-gamma ligands reduce inflammation and infarction size in transient focal ischemia. Neuroscience. 2005. 130:685–696.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Pioglitazone in Reducing Atherosclerosis Progression and Neointima Volume in Type 2 Diabetic Patients: Prospective Randomized Study With Volumetric Intravascular Ultrasonography Analysis
- Retrospective Observational Dose-Titration Study of Subjects with Type 2 Diabetes Mellitus with Inadequate Glycemic Control on 15 mg of Pioglitazone
- The Risk of Bladder Cancer in Korean Diabetic Subjects Treated with Pioglitazone
- Effects of Vildagliptin or Pioglitazone on Glycemic Variability and Oxidative Stress in Patients with Type 2 Diabetes Inadequately Controlled with Metformin Monotherapy: A 16-Week, Randomised, Open Label, Pilot Study
- Long-term Effect of Pioglitazone Treatment in Patients with Type 2 Diabetes