Korean J Urol.
2012 Sep;53(9):625-631. 10.4111/kju.2012.53.9.625.
Palliative Care of Malignant Ureteral Obstruction with Polytetrafluoroethylene Membrane-Covered Self-Expandable Metallic Stents: Initial Experience
- Affiliations
-
- 1Department of Urology, Korea Cancer Center Hospital, Seoul, Korea. jwpark@kcch.re.kr
- KMID: 2061701
- DOI: http://doi.org/10.4111/kju.2012.53.9.625
Abstract
- PURPOSE
We assessed the efficacy and safety of insertion of a polytetrafluoroethylene membrane-covered self-expandable metallic stent (UVENTA stent) for palliation of malignant ureteral obstruction on the basis of our early results.
MATERIALS AND METHODS
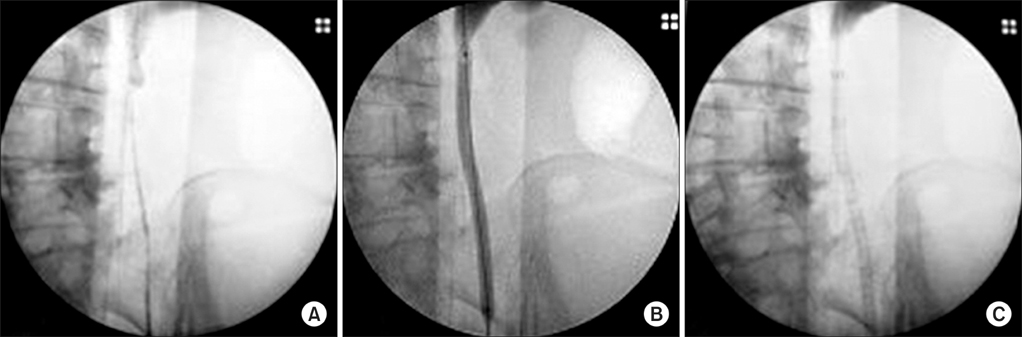
Eighteen patients underwent UVENTA stent insertion for extrinsic malignant ureteral obstructions of 20 ureters. The UVENTA stents were deployed retrogradely under cystoscopy and fluoroscopy. Candidates for the procedure had preexisting double-J stents that were nonfunctional or caused excessive bladder irritation. We recorded the success and patency rate in addition to any complications associated with the procedure.
RESULTS
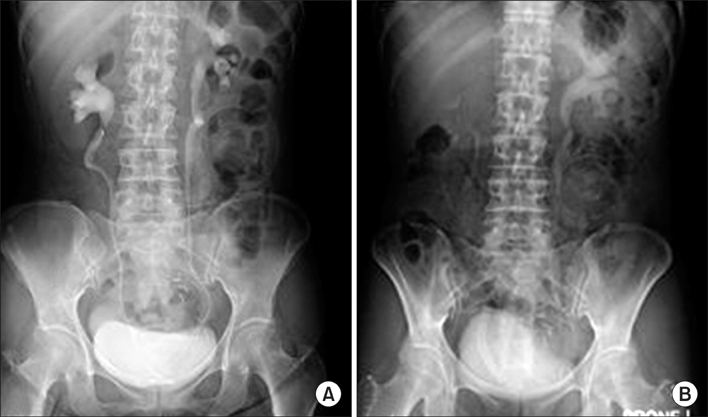
The mean length of obstruction was 10.6 cm (range, 2 to 20 cm). Two ureters were obstructed in the upper ureter, 9 in the lower ureter, and 9 in multiple levels of ureter. Simultaneous balloon dilation was performed in 12 ureters. UVENTA stents were successfully inserted in all patients. No obstruction of the UVENTA stents occurred during the mean follow-up period of 7.3 months (patency rate 100%), but de novo ureteral obstruction developed in 4 ureters. There were no instances of stone formation, hyperplastic reaction, encrustation, or migration. Abnormally elevated serum creatinine decreased to normal levels and hydronephrosis gradually resolved during the 4 weeks after UVENTA insertion. No significant complications developed except for transient and self-limiting hematuria and mild lower abdominal pain.
CONCLUSIONS
UVENTA stents may relieve malignant ureteral obstruction safely and easily. Long-term follow-up is necessary to assess the role of this stent in the treatment of malignant ureteral obstruction.
Keyword
MeSH Terms
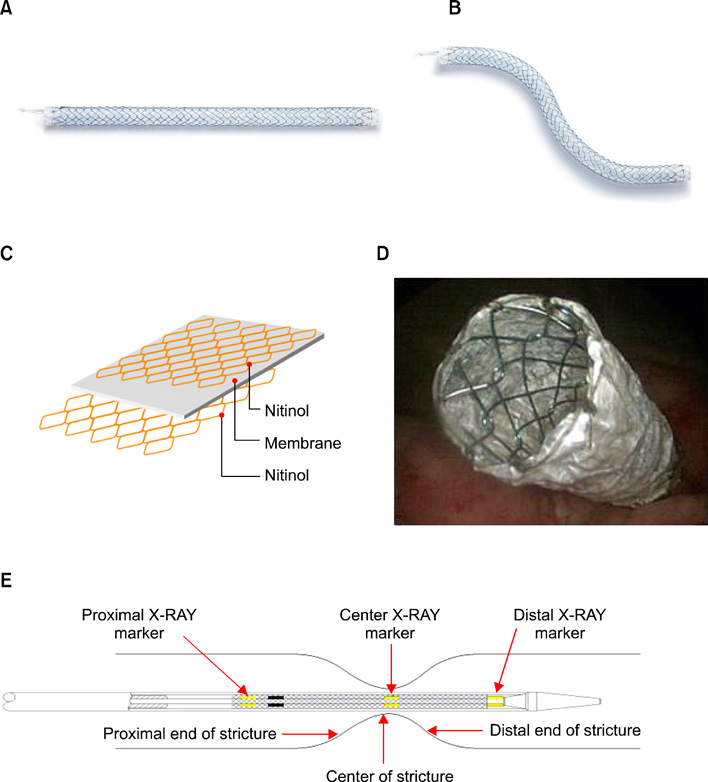
Figure
Cited by 1 articles
-
A Prospective Randomized Comparison of a Covered Metallic Ureteral Stent and a Double-J Stent for Malignant Ureteral Obstruction
Jong Woo Kim, Bumsik Hong, Ji Hoon Shin, Jihong Park, Jin Hyoun Kim, Dong Il Gwon, Min-Hee Ryu, Baek-Yeol Ryoo
Korean J Radiol. 2018;19(4):606-612. doi: 10.3348/kjr.2018.19.4.606.
Reference
-
1. Yachia D. Recent advances in ureteral stents. Curr Opin Urol. 2008. 18:241–246.2. Chung SY, Stein RJ, Landsittel D, Davies BJ, Cuellar DC, Hrebinko RL, et al. 15-year experience with the management of extrinsic ureteral obstruction with indwelling ureteral stents. J Urol. 2004. 172:592–595.3. Russo P. Urologic emergencies in the cancer patient. Semin Oncol. 2000. 27:284–298.4. Liatsikos EN, Karnabatidis D, Kagadis GC, Katsakiori PF, Stolzenburg JU, Nikiforidis GC, et al. Metal stents in the urinary tract. EAU-EBU Update Series. 2007. 5:77–88.5. Emmert C, Rassler J, Kohler U. Survival and quality of life after percutaneous nephrostomy for malignant ureteric obstruction in patients with terminal cervical cancer. Arch Gynecol Obstet. 1997. 259:147–151.6. Aravantinos E, Anagnostou T, Karatzas AD, Papakonstantinou W, Samarinas M, Melekos MD. Percutaneous nephrostomy in patients with tumors of advanced stage: treatment dilemmas and impact on clinical course and quality of life. J Endourol. 2007. 21:1297–1302.7. Russo P. Ureteral obstruction and stents: still a difficult problem for patients and urologists alike. J Urol. 2005. 174:2088.8. Ganatra AM, Loughlin KR. The management of malignant ureteral obstruction treated with ureteral stents. J Urol. 2005. 174:2125–2128.9. Watkinson AF, A'Hern RP, Jones A, King DM, Moskovic EC. The role of percutaneous nephrostomy in malignant urinary tract obstruction. Clin Radiol. 1993. 47:32–35.10. Lau MW, Temperley DE, Mehta S, Johnson RJ, Barnard RJ, Clarke NW. Urinary tract obstruction and nephrostomy drainage in pelvic malignant disease. Br J Urol. 1995. 76:565–569.11. Finney RP. Experience with new double J ureteral catheter stent. J Urol. 1978. 120:678–681.12. Hepperlen TW, Mardis HK, Kammandel H. The pigtail ureteral stent in the cancer patient. J Urol. 1979. 121:17–18.13. Gillams A, Dick R, Dooley JS, Wallsten H, el-Din A. Self-expandable stainless steel braided endoprosthesis for biliary strictures. Radiology. 1990. 174:137–140.14. Burgos FJ, Pascual J, Marcen R, Garcia-Navas R, Garcia IG, Alarcon C, et al. Self-expanding metallic ureteral stents for treatment of ureteral stenosis after kidney transplantation. Transplant Proc. 2005. 37:3828–3829.15. Liatsikos EN, Karnabatidis D, Katsanos K, Kallidonis P, Katsakiori P, Kagadis GC, et al. Ureteral metal stents: 10-year experience with malignant ureteral obstruction treatment. J Urol. 2009. 182:2613–2617.16. Barbalias GA, Liatsikos EN, Kalogeropoulou C, Karnabatidis D, Zabakis P, Athanasopoulos A, et al. Externally coated ureteral metallic stents: an unfavorable clinical experience. Eur Urol. 2002. 42:276–280.17. Agrawal S, Brown CT, Bellamy EA, Kulkarni R. The thermo-expandable metallic ureteric stent: an 11-year follow-up. BJU Int. 2009. 103:372–376.18. Chung HH, Lee SH, Cho SB, Park HS, Kim YS, Kang BC, et al. Comparison of a new polytetrafluoroethylene-covered metallic stent to a noncovered stent in canine ureters. Cardiovasc Intervent Radiol. 2008. 31:619–628.19. Arya M, Mostafid H, Patel HR, Kellett MJ, Philp T. The self-expanding metallic ureteric stent in the long-term management of benign ureteric strictures. BJU Int. 2001. 88:339–342.20. Klarskov P, Nordling J, Nielsen JB. Experience with Memokath 051 ureteral stent. Scand J Urol Nephrol. 2005. 39:169–172.21. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004. 240:205–213.22. Tekin MI, Aytekin C, Aygun C, PeSkircioglu L, Boyvat F, Ozkardes H. Covered metallic ureteral stent in the management of malignant ureteral obstruction: preliminary results. Urology. 2001. 58:919–923.23. Trueba Arguinarena FJ, Fernandez del Busto E. Self-expanding polytetrafluoroethylene covered nitinol stents for the treatment of ureteral stenosis: preliminary report. J Urol. 2004. 172:620–623.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Malignant Gastric Outlet Obstructions: Treatment with Self-Expandable Metallic Stents
- A Case of Pyloric Obstruction Caused by Self-expandable Metallic Stent for Palliation of Malignant Dysphagia
- Self-Expandable Metallic Stent Placement in the Palliative Treatment of Malignant Obstruction of Gastric Outlet and Duodenum
- Percutaneous placement of self-expandable metallic stents in patients with obstructive jaundice due to hepatocellular carcinoma
- Recent Advances in Ureteral Stents