J Korean Neurosurg Soc.
2014 May;55(5):237-243. 10.3340/jkns.2014.55.5.237.
Matrix Degradative Enzymes and Their Inhibitors during Annular Inflammation: Initial Step of Symptomatic Intervertebral Disc Degeneration
- Affiliations
-
- 1Department of Neurosurgery, Guro Hospital, College of Medicine, Korea University, Seoul, Korea. nskjh94@korea.ac.kr
- KMID: 2018027
- DOI: http://doi.org/10.3340/jkns.2014.55.5.237
Abstract
OBJECTIVE
Symptomatic disc degeneration develops from inflammatory reactions in the annulus fibrosus (AF). Although inflammatory mediators during annular inflammation have been studied, the roles of matrix metalloproteinases (MMPs) and their inhibitors have not been fully elucidated. In this study, we evaluated the production of MMPs and tissue inhibitors of metalloproteinase (TIMPs) during annular inflammation using an in vitro co-culture system. We also examined the effect of notochordal cells on annular inflammation.
METHODS
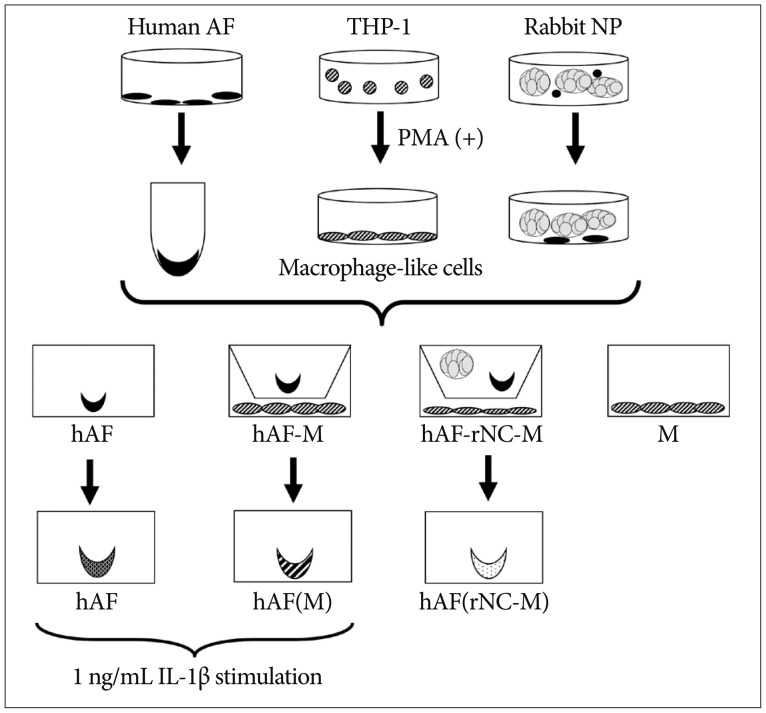
Human AF (hAF) pellet was co-cultured for 48 hours with phorbol myristate acetate-stimulated macrophage-like THP-1 cells. hAF pellet and conditioned media (CM) from co-cultured cells were assayed for MMPs, TIMPs, and insulin-like growth factor (IGF)-1 levels using real-time reverse-transcriptase polymerase chain reaction and enzyem-linked immunosorbent assay. To evaluate whether notochordal cells affected MMPs or TIMPs production on annular inflammation, hAF co-cultured with notochordal cells from adult New Zealand White rabbits, were assayed.
RESULTS
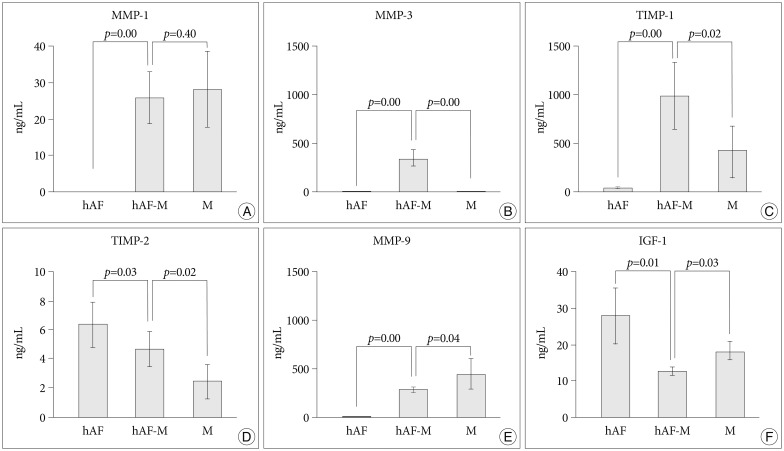
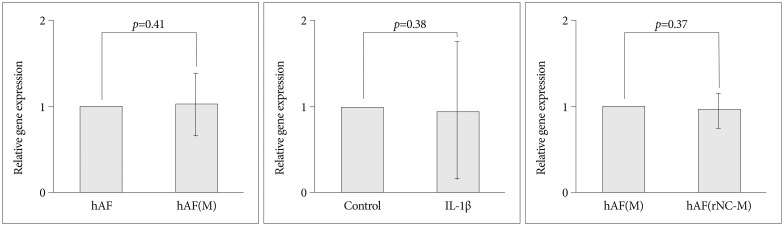
MMP-1, -3, -9; and TIMP-1 levels were significantly increased in CM of hAF co-cultured with macrophage-like cells compared with hAF alone, whereas TIMP-2 and IGF-1 levels were significantly decreased (p<0.05). After macrophage exposure, hAF produced significantly more MMP-1 and -3 and less TIMP-1 and -2. Interleukin-1beta stimulation enhanced MMP-1 and -3 levels, and significantly diminished TIMP-2 levels. Co-culturing with rabbit notochordal cells did not significantly influence MMPs and TIMPs production or COL1A2 gene expression.
CONCLUSION
Our results indicate that macrophage-like cells evoke annular degeneration through the regulation of major degradative enzymes and their inhibitors, produced by hAF, suggesting that the selective regulation of these enzymes provides future targets for symptomatic disc degeneration therapy.
Keyword
MeSH Terms
-
Adult
Coculture Techniques
Culture Media, Conditioned
Gene Expression
Humans
Inflammation*
Insulin-Like Growth Factor I
Interleukin-1beta
Intervertebral Disc Degeneration*
Macrophages
Matrix Metalloproteinases
Myristic Acid
Notochord
Polymerase Chain Reaction
Rabbits
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Culture Media, Conditioned
Insulin-Like Growth Factor I
Interleukin-1beta
Matrix Metalloproteinases
Myristic Acid
Tissue Inhibitor of Metalloproteinase-1
Tissue Inhibitor of Metalloproteinase-2
Figure
Reference
-
1. Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells : regulation of proteoglycan synthesis. Exp Cell Res. 1999; 246:129–137. PMID: 9882522.
Article2. Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999; 354:581–585. PMID: 10470716.
Article3. Bachmeier BE, Nerlich A, Mittermaier N, Weiler C, Lumenta C, Wuertz K, et al. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J. 2009; 18:1573–1586. PMID: 19466462.
Article4. Gomez-Camarillo MA, Almonte-Becerril M, Vasquez Tort M, Tapia-Ramirez J, Kouri Flores JB. Chondrocyte proliferation in a new culture system. Cell Prolif. 2009; 42:207–218. PMID: 19236380.
Article5. Kim JH, Deasy BM, Seo HY, Studer RK, Vo NV, Georgescu HI, et al. Differentiation of intervertebral notochordal cells through live automated cell imaging system in vitro. Spine (Phila Pa 1976). 2009; 34:2486–2493. PMID: 19841610.
Article6. Kim JH, Studer RK, Sowa GA, Vo NV, Kang JD. Activated macrophage-like THP-1 cells modulate anulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine (Phila Pa 1976). 2008; 33:2253–2259. PMID: 18784630.
Article7. Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004; 84:649–698. PMID: 15044685.
Article8. Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004; 204:47–54. PMID: 15307137.
Article9. Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008; 17(Suppl 4):441–451. PMID: 19005698.
Article10. Masuda K, Miyabayashi T, Meachum SH, Eurell TE. Proliferation of canine intervertebral disk chondrocytes in three-dimensional alginate microsphere culture. J Vet Med Sci. 2002; 64:79–82. PMID: 11853153.
Article11. Masuhara K, Nakai T, Yamaguchi K, Yamasaki S, Sasaguri Y. Significant increases in serum and plasma concentrations of matrix metalloproteinases 3 and 9 in patients with rapidly destructive osteoarthritis of the hip. Arthritis Rheum. 2002; 46:2625–2631. PMID: 12384920.
Article12. Moon HJ, Joe H, Kwon TH, Choi HK, Park YK, Kim JH. Notochordal cells influence gene expression of inflammatory mediators of annulus fibrosus cells in proinflammatory cytokines stimulation. J Korean Neurosurg Soc. 2010; 48:1–7. PMID: 20717505.
Article13. Murphy G, Knäuper V, Atkinson S, Butler G, English W, Hutton M, et al. Matrix metalloproteinases in arthritic disease. Arthritis Res. 2002; 4(Suppl 3):S39–S49. PMID: 12110122.14. Okuma M, Mochida J, Nishimura K, Sakabe K, Seiki K. Reinsertion of stimulated nucleus pulposus cells retards intervertebral disc degeneration : an in vitro and in vivo experimental study. J Orthop Res. 2000; 18:988–997. PMID: 11192261.
Article15. Peng B, Hao J, Hou S, Wu W, Jiang D, Fu X, et al. Possible pathogenesis of painful intervertebral disc degeneration. Spine (Phila Pa 1976). 2006; 31:560–566. PMID: 16508552.
Article16. Peng B, Wu W, Hou S, Li P, Zhang C, Yang Y. The pathogenesis of discogenic low back pain. J Bone Joint Surg Br. 2005; 87:62–67. PMID: 15686239.
Article17. Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase : their role in disorders of the human intervertebral disc. Spine (Phila Pa 1976). 2000; 25:3005–3013. PMID: 11145811.18. Roughley PJ. Biology of intervertebral disc aging and degeneration : involvement of the extracellular matrix. Spine (Phila Pa 1976). 2004; 29:2691–2699. PMID: 15564918.19. Stetler-Stevenson WG, Seo DW. TIMP-2 : an endogenous inhibitor of angiogenesis. Trends Mol Med. 2005; 11:97–103. PMID: 15760767.20. Tsuchiya S, Kobayashi Y, Goto Y, Okumura H, Nakae S, Konno T, et al. Induction of maturation in cultured human monocytic leukemia cells by a phorbol diester. Cancer Res. 1982; 42:1530–1536. PMID: 6949641.21. Walmsley R. The development and growth of the intervertebral disc. Edinb Med J. 1953; 60:341–364. PMID: 13083579.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biochemical Factors of Intervertebral Disc Degeneration: Implications for Disc Regeneration
- Current Knowledge and Future Therapeutic Prospects in Symptomatic Intervertebral Disc Degeneration
- Analysis of the Correlation Among Age, Disc Morphology, Positive Discography and Prognosis in Patients With Chronic Low Back Pain
- Neural Mechanisms of Discogenic Back Pain: How Does Nerve Growth Factor Play a Key Role?
- Macrophage Infiltrations and Expressions of Matrix Metallproteinases in Intervertebral Disc