J Korean Soc Radiol.
2013 Jun;68(6):489-498. 10.3348/jksr.2013.68.6.489.
Comparison of Radiologic Features of Triple-Negative and Estrogen Receptor/Progesteron Receptor Positive Breast Cancer
- Affiliations
-
- 1Department of Radiology, Konyang University College of Medicine, Konyang University Hospital, Daejeon, Korea. lizkim1@hanmail.net
- 2Department of Radiology, Sam Hospital, Anyang, Korea.
- 3Department of Nuclear Medicine, Konyang University College of Medicine, Konyang University Hospital, Daejeon, Korea.
- 4Department of General Surgery, Konyang University College of Medicine, Konyang University Hospital, Daejeon, Korea.
- 5Department of Radiology, Eulji University College of Medicine, Eulji University Hospital, Daejeon, Korea.
- 6Myeonggok Medical Research Institute, Konyang University College of Medicine, Daejeon, Korea.
- KMID: 2002901
- DOI: http://doi.org/10.3348/jksr.2013.68.6.489
Abstract
- PURPOSE
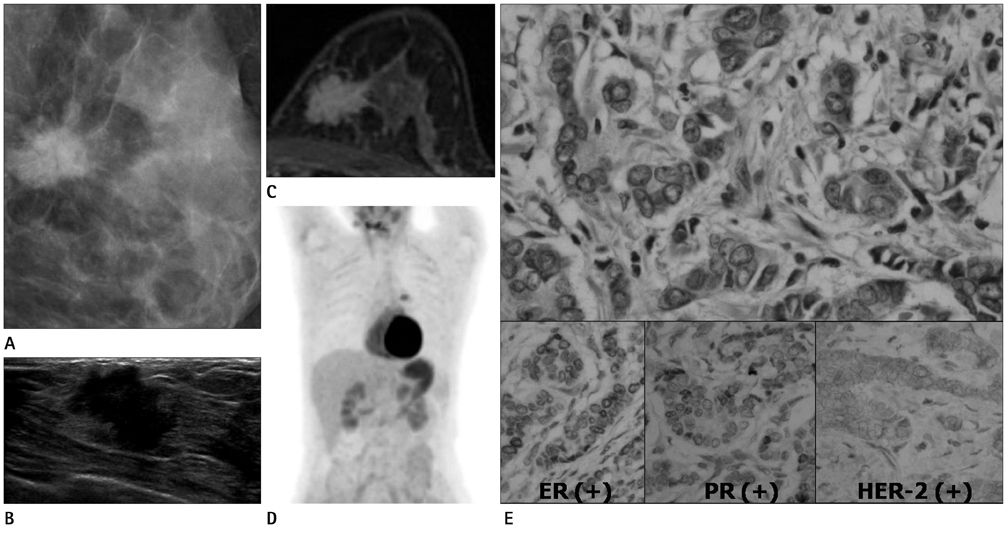
To retrospectively investigate the imaging [mammographic, ultrasonographic (US), magnetic resonance (MR) imaging] features and standardized uptake values (SUV) in positron emission tomography (PET)/computed tomography (CT) of triple-negative breast cancers (TNBC) and to compare them with breast cancers that are either estrogen receptor (ER) positive or progesteron receptor (PR) positive.
MATERIALS AND METHODS
155 breast cancers cases were identified in 134 women (mean age, 51 years; range, 31-86 years). Surgically confirmed TNBC (n = 27) and ER-positive/PR-positive breast cancers (n = 81) were included among them. Cancers were investigated with mammography (n = 81), US (n = 106), MR imaging (n = 34) and PET-CT (n = 59). Mammographic findings are identified by detection of characteristic masses and microcalcifications. US findings included tumor size, margin, tumor shape, calcification and posterior shadowing. MR findings included tumor size, shape, margin, internal enhancement, intratumoral signal intensity and kinetics. Peak SUVs (p-SUV) of breast cancers were evaluated in PET/CT. These findings were compared with TNBC and ER/PR positive groups.
RESULTS
Mammographic findings had no significant association with the TNBC. High pathological grade (p < 0.05), larger than 2 cm in size, well-marginal mass, and round or oval-shaped (p < 0.05) is US were significantly associated with TNBC. In MR imaging, round mass shape (p < 0.05), well-circumscribed mass margin (p < 0.05), rim enhancement (p < 0.05), were significantly associated with TNBC. The peak SUV of TNBC tend to be higher than that of ER-positive/PR-positive breast cancer (7.95 +/- 5.50 vs. 4.91 +/- 3.00, p < 0.05).
CONCLUSION
TNBC tend to have high pathological grade, are of a large, round and smooth mass with rim enhancement on MR and US. In addition to above features, PET-CT with SUV estimation can improve the accuracy of test through the evaluation of TNBC.
MeSH Terms
Figure
Reference
-
1. Lee WC, Park JS, Lee JH. Screening of breast cancer. Korean Society of Breast imaging. Breast diagnostic imaging. 2nd ed. Seoul: Ilchokak;2012. p. 153–164.2. Glass AG, Lacey JV Jr, Carreon JD, Hoover RN. Breast cancer incidence, 1980-2006: combined roles of menopausal hormone therapy, screening mammography, and estrogen receptor status. J Natl Cancer Inst. 2007; 99:1152–1161.3. Ravdin PM, Cronin KA, Howlader N, Berg CD, Chlebowski RT, Feuer EJ, et al. The decrease in breast-cancer incidence in 2003 in the United States. N Engl J Med. 2007; 356:1670–1674.4. Sørlie T. Molecular portraits of breast cancer: tumour subtypes as distinct disease entities. Eur J Cancer. 2004; 40:2667–2675.5. Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: a critical review. J Clin Oncol. 2008; 26:2568–2581.6. Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008; 52:108–118.7. Haffty BG, Yang Q, Reiss M, Kearney T, Higgins SA, Weidhaas J, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006; 24:5652–5657.8. American college of radiology. Breast imaging reading and data system, Breast imaging atlas. 4th ed. Reston, VA: American Collegeof Radiology;2003.9. Peto R, Boreham J, Clarke M, Davies C, Beral V. UK and USA breast cancer deaths down 25% in year 2000 at ages 20-69 years. Lancet. 2000; 355:1822.10. Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005; 353:1673–1684.11. Gonzalez-Angulo AM, Hortobágyi GN, Esteva FJ. Adjuvant therapy with trastuzumab for HER-2/neu-positive breast cancer. Oncologist. 2006; 11:857–867.12. Tokunaga E, Oki E, Nishida K, Koga T, Egashira A, Morita M, et al. Trastuzumab and breast cancer: developments and current status. Int J Clin Oncol. 2006; 11:199–208.13. Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007; 109:25–32.14. Rodenhuis S, Bontenbal M, van Hoesel QG, Smit WM, Nooij MA, Voest EE, et al. Efficacy of high-dose alkylating chemotherapy in HER2/neu-negative breast cancer. Ann Oncol. 2006; 17:588–596.15. De Giorgi U, Rosti G, Frassineti L, Kopf B, Giovannini N, Zumaglini F, et al. High-dose chemotherapy for triple negative breast cancer. Ann Oncol. 2007; 18:202–203.16. Wang Y, Ikeda DM, Narasimhan B, Longacre TA, Bleicher RJ, Pal S, et al. Estrogen receptor-negative invasive breast cancer: imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression. Radiology. 2008; 246:367–375.17. Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009; 250:638–647.18. Stavros AT, Rapp CL, Kaske TI, Parker SH. Hard and soft sonographic findings of malignancy. In : Feig SA, editor. Categorical course in diagnostic radiology: breast imaging. Chicago, IL: Radiological Society of North America;2005. p. 125–142.19. Kuhl CK, Schild HH. Dynamic image interpretation of MRI of the breast. J Magn Reson Imaging. 2000; 12:965–974.20. Beliën JA, van Diest PJ, Baak JP. Relationships between vascularization and proliferation in invasive breast cancer. J Pathol. 1999; 189:309–318.21. Teifke A, Behr O, Schmidt M, Victor A, Vomweg TW, Thelen M, et al. Dynamic MR imaging of breast lesions: correlation with microvessel distribution pattern and histologic characteristics of prognosis. Radiology. 2006; 239:351–360.22. Dogan BE, Gonzalez-Angulo AM, Gilcrease M, Dryden MJ, Yang WT. Multimodality imaging of triple receptor-negative tumors with mammography, ultrasound, and MRI. AJR Am J Roentgenol. 2010; 194:1160–1166.23. Mavi A, Cermik TF, Urhan M, Puskulcu H, Basu S, Yu JQ, et al. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions. J Nucl Med. 2007; 48:1266–1272.24. Basu S, Chen W, Tchou J, Mavi A, Cermik T, Czerniecki B, et al. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for disease characterization. Cancer. 2008; 112:995–1000.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment Outcomes of Weakly Positive Hormone Receptor Breast Cancer and Triple-Negative Breast Cancer
- Estrogen Receptor alpha, beta and Progesteron Receptor Expression in Gynecomastia Using Immunohistochemical Staining
- Clinicopathologic Characteristics and Prognosis of Early Stage Triple Negative Breast Cancer: Comparison with Non-triple Negative Group
- MRI Findings of Triple Negative Breast Cancer: A Comparison with Non-Triple Negative Breast Cancer
- Clinical Differences in Triple-Positive Operable Breast Cancer Subtypes in Korean Patients: An Analysis of Korean Breast Cancer Registry Data