Korean J Obstet Gynecol.
2012 Jul;55(7):507-512. 10.5468/KJOG.2012.55.7.507.
A Case of Recurrent Ovarian Granulosa Cell Tumor Associated with Sarcomatoid Change
- Affiliations
-
- 1Department of Obstetrics, Yeungnam University School of Medicine, Daegu, Korea. djlee@med.yu.ac.kr
- 2Department of Pathology, Yeungnam University School of Medicine, Daegu, Korea.
- KMID: 1992561
- DOI: http://doi.org/10.5468/KJOG.2012.55.7.507
Abstract
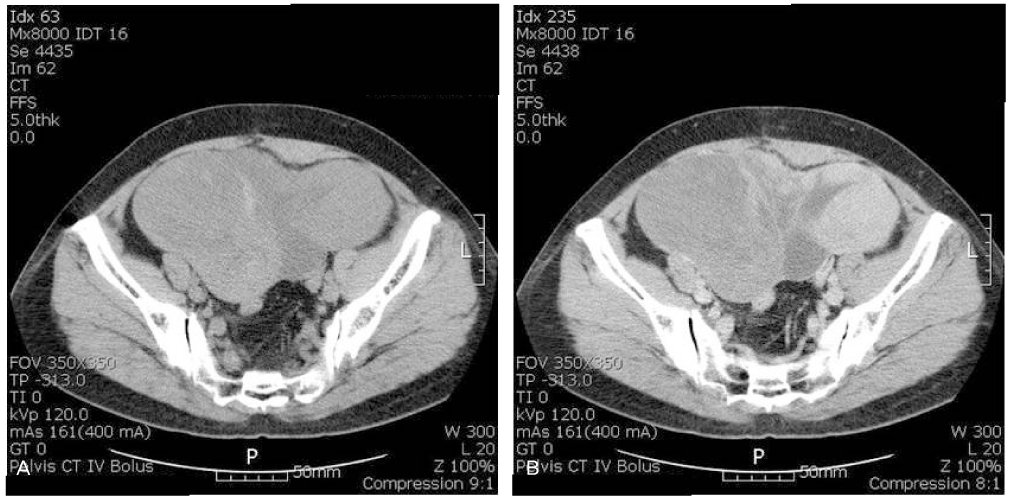
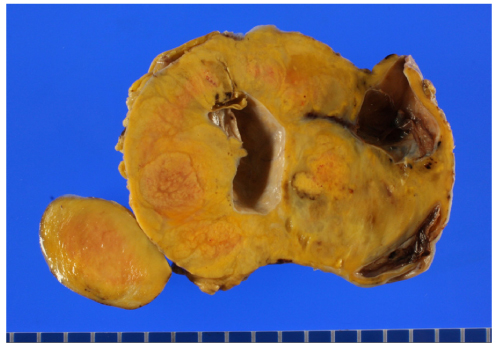
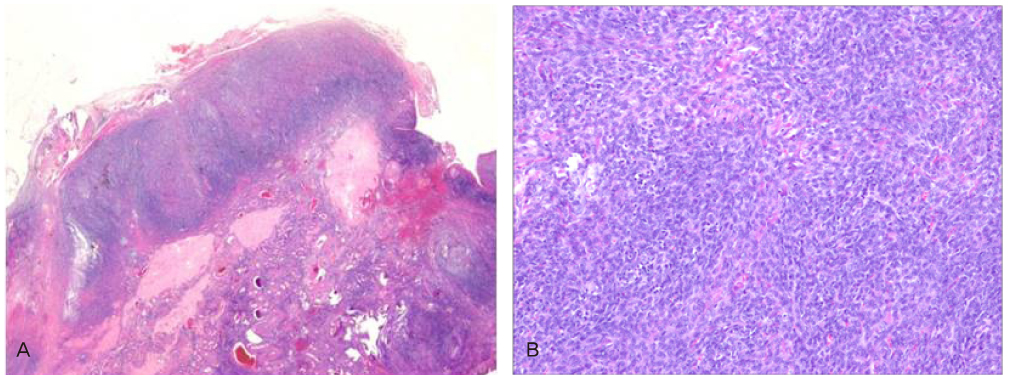
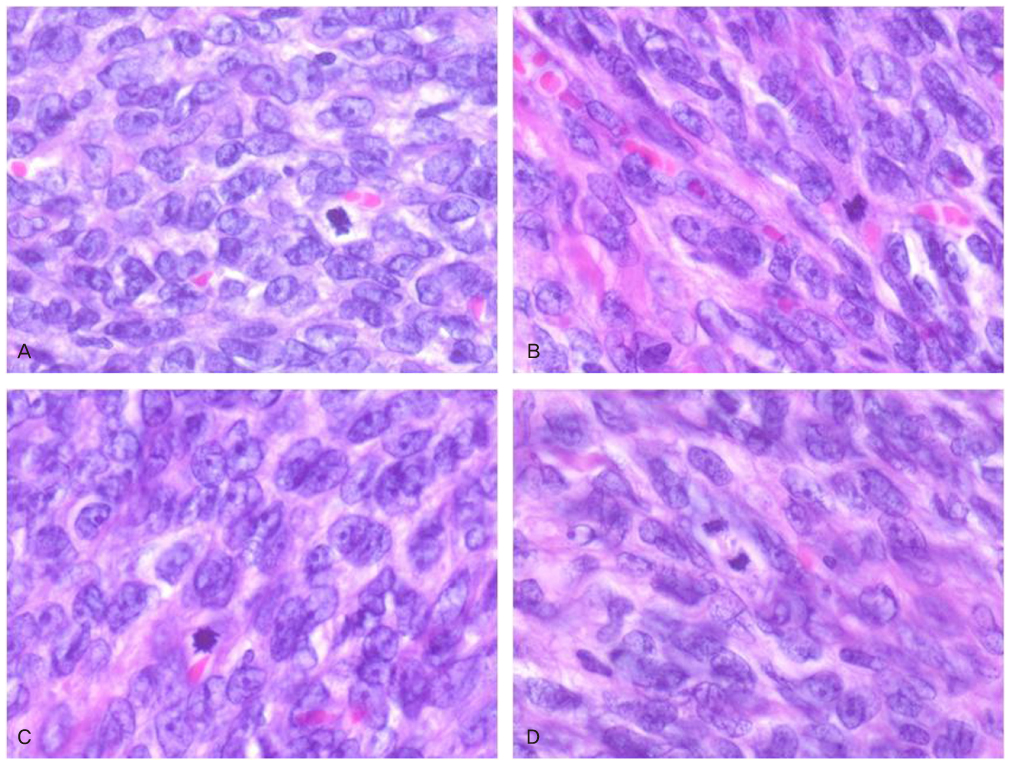
- Granulosa cell tumors of the ovary are malignancies that originate from the sex-cord stromal cells of the ovary and represent 2% to 5% of all ovarian cancers. Slow growth with a tendency for late recurrence characterizes a natural history of this tumor. So this tumor needs a prolonged follow-up. As a result of recent literature in Korea, the findings are 3 cases of juvenile granulosa cell tumor and 1 case of adult granulosa cell tumor. Previous case of adult granulosa cell tumor was presented recurrence with hepatic metastasis after 9 months of first diagnosis and operation. We describe here a 52-year-old women with recurrent granulosa cell tumor after total abdominal hysterectomy with right salpingo-oophorectomy and left ovarian wedge resection because of right ovarian granulosa cell tumor 12 years ago. Our case presented late recurrence character of granulosa cell tumor. We report 1 case of recurrent adult type granulosa cell tumor associated sarcomatoid change with a brief review of literatures.
Keyword
MeSH Terms
Figure
Reference
-
1. Schumer ST, Cannistra SA. Granulosa cell tumor of the ovary. J Clin Oncol. 2003. 21:1180–1189.2. Young RH, Dickersin GR, Scully RE. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol. 1984. 8:575–596.3. Fox H, Agrawal K, Langley FA. A clinicopathologic study of 92 cases of granulosa cell tumor of the ovary with special reference to the factors influencing prognosis. Cancer. 1975. 35:231–241.4. Geetha P, Nair MK. Granulosa cell tumours of the ovary. Aust N Z J Obstet Gynaecol. 2010. 50:216–220.5. Ko SF, Wan YL, Ng SH, Lee TY, Lin JW, Chen WJ, et al. Adult ovarian granulosa cell tumors: spectrum of sonographic and CT findings with pathologic correlation. AJR Am J Roentgenol. 1999. 172:1227–1233.6. Pectasides D, Pectasides E, Psyrri A. Granulosa cell tumor of the ovary. Cancer Treat Rev. 2008. 34:1–12.7. Shah VI, Freites ON, Maxwell P, McCluggage WG. Inhibin is more specific than calretinin as an immunohistochemical marker for differentiating sarcomatoid granulosa cell tumour of the ovary from other spindle cell neoplasms. J Clin Pathol. 2003. 56:221–224.8. Koukourakis GV, Kouloulias VE, Koukourakis MJ, Zacharias GA, Papadimitriou C, Mystakidou K, et al. Granulosa cell tumor of the ovary: tumor review. Integr Cancer Ther. 2008. 7:204–215.9. Nosov V, Silva I, Tavassoli F, Adamyan L, Farias-Eisner R, Schwartz PE. Predictors of recurrence of ovarian granulosa cell tumors. Int J Gynecol Cancer. 2009. 19:628–633.10. Uygun K, Aydiner A, Saip P, Basaran M, Tas F, Kocak Z, et al. Granulosa cell tumor of the ovary: retrospective analysis of 45 cases. Am J Clin Oncol. 2003. 26:517–521.11. Woods DC, Alvarez C, Johnson AL. Cisplatin-mediated sensitivity to TRAIL-induced cell death in human granulosa tumor cells. Gynecol Oncol. 2008. 108:632–640.12. Chua TC, Iyer NG, Soo KC. Prolonged survival following maximal cytoreductive effort for peritoneal metastases from recurrent granulosa cell tumor of the ovary. J Gynecol Oncol. 2011. 22:214–217.13. Yoon JH, Chang SJ, Chang KH, Ryu HS. Clinicopathologic characteristics of granulosa cell tumor of the ovary. Korean J Gynecol Oncol. 2007. 18:172–179.14. Lee JN, Jung MH, Yoo HJ, Kim DY, Kim JH, Kim YM, et al. A clinical study of ovarian granulosa cell tumor. Korean J Gynecol Oncol Colposc. 2004. 15:223–230.15. Park SG, Shin YS, Son SK, Nam SL, Seo KS, Kim SY. A case of hepatic metastasis in granulosa cell tumor of the ovary. Korean J Gynecol Oncol Colposc. 1998. 9:325–328.