J Korean Acad Conserv Dent.
2002 Mar;27(2):183-195. 10.5395/JKACD.2002.27.2.183.
Cytotoxic effects of prevotella nigrescens on cultured cells
- Affiliations
-
- 1Department of Conservative Dentistry, College of Dentistry, Wonkwang University, Korea.
- 2Department of Oral Biochemistry, College of Dentistry, Wonkwang University, Korea.
- 3Department of Oral Medicine, College of Dentistry, Wonkwang University, Korea.
- KMID: 1987277
- DOI: http://doi.org/10.5395/JKACD.2002.27.2.183
Abstract
- No abstract available.
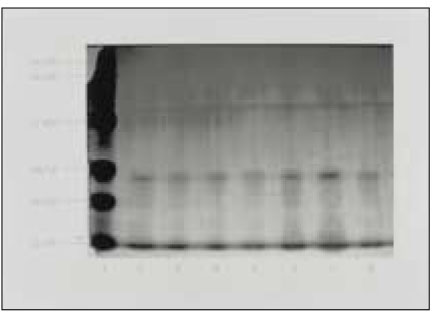
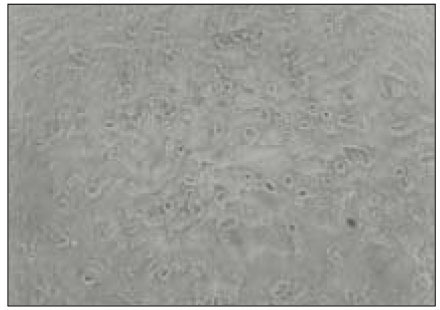
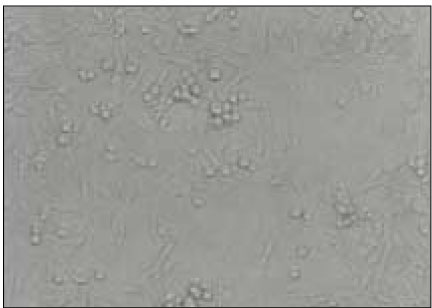
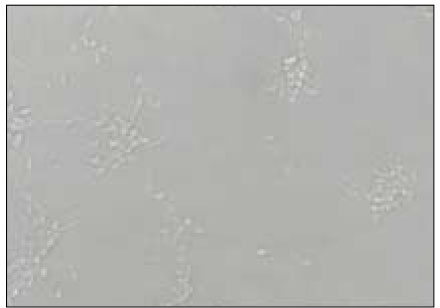
Figure
Reference
-
1. Korzen BH, Krakow AA, Green DB. Pulpal and periapical tissue responses in conventional and monoinfected gnotobiotic rats. Oral Surg Oral Med Oral Pathol. 1974. 37:783–802.
Article2. Farber P, Seltzer S. Endodontic microbiology. I. Etiology. J Endod. 1988. 14:363–371.
Article3. Sundqvist G. Taxonomy, ecology, and pathogenicity of the root canal flora. Oral Surg Oral Med Oral Pathol. 1994. 78:522–530.
Article4. Sundqvist G. Bacteriological studies of necrotic dental pulps. 1976. Umea, Sweden: Umea university;[thesis].5. Tani-Ishii N, Wang CY, Tanner A, Stashenko P. Changes in root canal microbiota during the development of rat periapical lesions. Oral Microbiol Immunol. 1994. 9:129–135.
Article6. van Winkelhoff AJ, Carlee AW, de Graaff J. Bacteroides endodontalis and other black-pigmented Bacteroides species in odontogenic abscesses. Infect Immun. 1985. 49:494–498.
Article7. Stashenko P, Teles R, D'Souza R. Periapical inflammatory responses and their modulation. Crit Rev Oral Biol Med. 1998. 9:498–521.
Article8. Stashenko P. Regulatory effect of monocytes on T cell proliferative responses to oral microbial antigens. Infect Immun. 1982. 38:938–947.
Article9. Torabinejad M, Clagett J, Engel D. A cat model for evaluation of mechanisms of bone resorption: induction of bone loss by simulated immune complexes and inhibition by indomethacin. Calcif Tissue Int. 1979. 29:207–214.
Article10. Shah HN, Collins DM. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int J Syst Bacteriol. 1990. 40:205–208.
Article11. Gharbia SE, Haapasalo M, Shah HN, Kotiranta A, Lounatmaa K, Pearce MA, Devine DA. Characterization of Prevotella intermedia and Prevotella nigrescens isolates from periodontic and endodontic infections. J Periodontol. 1994. 65:56–61.
Article12. Baumgartner JC, Watkins BJ, Bae KS, Xia T. Association of black-pigmented bacteria with endodontic infections. J Endod. 1999. 25:413–415.
Article13. Johansson A, Bergenholtz A, Holm SE. Strong cytotoxicity to human gingival fibroblasts by Porphyromonas gingivalis ATCC 33277. J Periodont Res. 1996. 31:477–482.
Article14. Yamasaki M, Nakata K, Imaisumi I, Iwama A, Nakane A, Nakamura H. Cytotoxic effect of endodontic bacteria on periapical fibroblasts. J Endod. 1998. 24:534–539.
Article15. Browne RM. The in vitro assessment of the cytotoxicity of dental materials-does it have a role? Int Endod J. 1988. 21:50–58.
Article16. Baumgartner JC, Watkins BJ, Bae K-S, Xia T. Assoication of black-pigmented bacteria with endodontic infections. J Endod. 1999. 25:413–415.17. Eidhin DN, Mouton C. A rapid method for preparation of rough and smooth lipopolysaccjaride from Bacteroides, Porphyromonas and Prevotella. FEMS Microbiol Lett. 1993. 110:133–138.
Article18. Sundqvist G, Johansson E, Sjogren U. Prevalence of black-pigmented bacteria with endodontic infections. J Endod. 1989. 15:13–19.19. Frandsen EVG, Poulsen K, Kilian M. Confirmation of the species Prevotella intermedia and Prevotella nigrescens. Int J Syst Bacteriol. 1995. 45:429–435.
Article20. Bae KS, Baumgartner JC, Shearer TR, David LL. Occurrence of Prevotella nigrescens and Prevotella intermedia in infections of endodontic origin. J Endod. 1997. 23:620–623.
Article21. van Steenbergen T, Ouden D, Touw JA, Graarr JD. Cytotoxic activity of Bacteroides gingivalis and Bacteroides asaccharolyticus. J Med Microbiol. 1982. 5:253–258.
Article22. Shah HN, Gharbia SE, O'Toole CM. Assessment of the relative cytotoxicity of Porphyromonas gingivalis cells, products, and components on human epithelial cell lines. J Periodontol. 1992. 63:44–51.
Article23. Larjava H, Uitto VJ. Effects of extracts from Bacteroides gingivalis, Bacteroides intermedius, and Bacteroides asaccharolyticus on the growth of fibroblast lines obtained from healthy and inflamed human gingiva. Oral Microbiol Immunol. 1987. 2:112–116.
Article24. Stevens RH, Hammond BF. The comparative cytotoxicity of periodontal bacteria. J Periodontol. 1988. 59:741–749.
Article25. Pissiotis E, Spangberg LSW. Toxicity of sonicated extracts of Bacteroides gingivalis on human pulpal cells and L929 cells in vitro. J Endod. 1991. 17:553–560.
Article26. Hausmann E, Weinfeld N, Miller WA. Effects of lipopolysaccharides on bone resorption in tissue culture. Calcif Tissue Res. 1972. 9:272–282.
Article27. Schein B, Schilder H. Endotoxin content in endodontically involved teeth. J Endod. 1975. 1:19–21.
Article28. Horiba N, Maekawa Y, Yamauchi Y, Ito M, Matsumoto T, Nakamura H. Complement activation by lipopolysaccharides purified from gram-negative bacteria isolated from infected root canals. Oral Surg Oral Med Oral Pathol. 1992. 74:648–651.
Article29. Coutingho A, Moller G. Thymus-independent B-cell induction and paralysis. Adv Immunol. 1975. 21:113–136.30. Burchett SK, Weaver WM, Westall JA, Larsen A, Kronheim S, Wilson CB. Regulation of tumor necrosis factor/cachecin and IL-1 secretion in human mononuclear phagocytes. J Immunol. 1988. 140:3473–3481.31. Melchers F, Braun V, Galanos C. The lipoprotein of the outer membrane of Escherichia coli: Ab-lymphocyte mitogen. J Exp Med. 1975. 142:473–482.
Article32. Dewhirst FE. N-acetyl muramyl dipeptide stimulation of bone resorption in tissue culture. Infect Immun. 1982. 35:133–137.
Article33. Morrison DC, Betz SJ, Jacobs DM. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976. 144:840–846.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interleukin-8 production and interleukin-8 mRNA expression induced by lipopolysaccharides from Prevotella intermedia and Prevotella nigrescens in monocyte-derived macrophages
- Chemical and Immunobiological Characterization of Lipopolysaccharides from Prevotella intermedia and Prevotella nigrescens
- The Frequency of Detecting Prevotella intermedia and Prevotella nigrescens in Korean Adult Periodontitis Patients
- Isolation and Partial Characterization of Hemin-binding Cell Envelope Proteins from Porphyromonas gingivalis, Prevotella intermedia, and Prevotella nigrescens
- A study on the hemolytic properties of Prevotella nigrescens