Tuberc Respir Dis.
2006 Sep;61(3):239-247. 10.4046/trd.2006.61.3.239.
The Purification and Immunogenicity of TB-14 Recombinant Protein of Mycobacterium tuberculosis
- Affiliations
-
- 1Department of Microbiology, College of Medicine, Soonchunhyang University, Cheonan, Korea. songmic@sch.ac.kr
- 2Department of Physiology, College of Medicine, Soonchunhyang University, Cheonan, Korea.
- 3Department of Pathology, Cheongju St. Mary Hospital, Cheongju City, Chungbuk, Korea.
- KMID: 1970263
- DOI: http://doi.org/10.4046/trd.2006.61.3.239
Abstract
-
BACKGROUND: Culture filtrate proteins secreted by mycobacteria are thought to play an important role in inducing protective immunity and to develop new methods for diagnosing tuberculosis.
METHODS
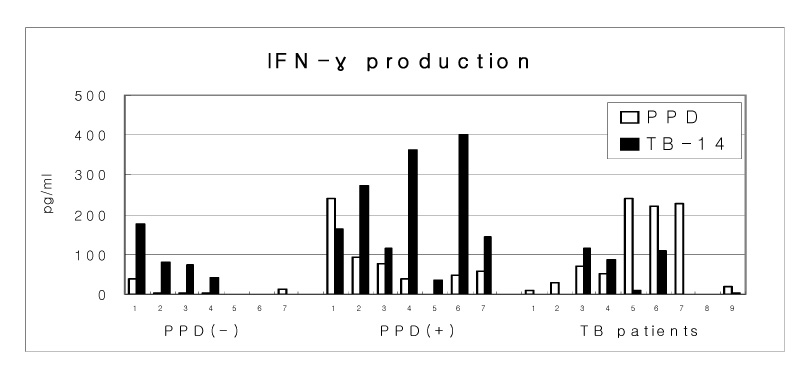
A culture filtrate protein of M. avium that was strongly reactive with goat antiserum against M. intracellulare was constructed. Its homologous protein (TB-14) in M. tuberculosis was cloned, expressed and purified. The inductions of IFN-gamma stimulated with 10 microgram of TB-14 recombinant protein and 10 microgram PPD were estimated by using whole bloods from seven PPD (-) subjects, seven PPD (+) healthy volunteers and nine tuberculosis patients.
RESULTS
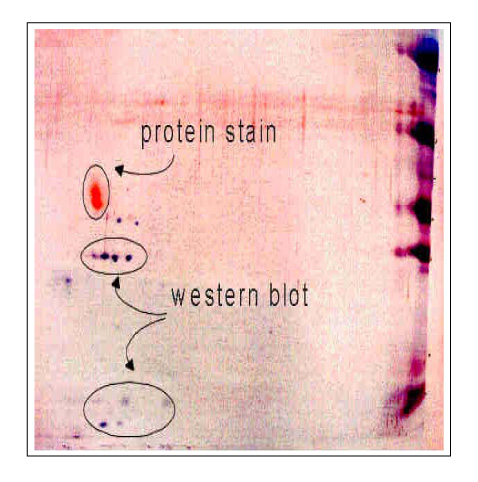
M. avium culture filtrate protein was confirmed as a hypothetical protein that was termed contig 116. A novel 14-kDa recombinant protein (TB-14) of M. tuberculosis was composed of 148 amino acids, including 30 amino acids of the signal peptide, and it showed 78% homology with M. avium. In the PPD (+) healthy volunteers, recombinant TB-14 protein strongly induced the secretion of IFN-gamma in whole blood cultures.
CONCLUSION
These results suggest that TB-14 recombinant protein might play an important role in inducing cell-mediated immunity against tuberculosis. Furthermore, TB-14 protein antigen and its antiserum will be available for the development of new diagnostic tools for tuberculosis.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. Global tuberculosis programme: the TB treatment observer. WHO Newsletter. 1997.2. World Health Organization. WHO declares tuberculosis a global emergency. Soz Praventivmed. 1993. 38:251–252.3. WHO report on the tuberculosis epidemic. World Health Organization. Avail from: http://www.who.ch/.4. Boesen H, Jensen BN, Wilcke T, Andersen P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995. 63:1491–1497.5. Orme IM, Andersen P, Boom WH. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993. 167:1481–1497.6. Roberts AD, Sonnenberg MG, Ordway DJ, Furney SK, Brennan PJ, Belisle JT, et al. Characteristics of protective immunity engendered by vaccination of mice with purified culture filtrate protein antigens of Mycobacterium tuberculosis. Immunology. 1995. 85:502–508.7. Young DB, Kaufmann SH, Hermans PW, Thole JE. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992. 6:133–145.8. Ison CA, Anwer N, Cole MJ, Galassini R, Heyderman RS, Klein NJ, et al. Assessment of immune response to meningococcal disease: comparison of a whole-blood assay and the serum bactericidal assay. Microb Pathog. 1999. 27:207–214.9. Miles AA, Misra SS. The estimation of the bactericidal power of the blood. J Hyg. 1938. 38:732–749.10. Wallis RS, Lederman HM, Spritzler J, Devers JL, Georges D, Weinberg A, et al. Measurement of induced cytokines in AIDS clinical trials using whole blood: a preliminary report. Clin Diagn Lab Immunol. 1998. 5:556–560.11. Wallis RS, Palaci M, Vinhas S, Hise AG, Ribeiro FC, landen K, et al. A whole blood bactericidal assay for tuberculosis. J Infect Dis. 2001. 183:1300–1303.12. Bose M, Chander A, Das RH. A rapid and gentle method for the isolation of genomic DNA from mycobacteria. Nucleic Acids Res. 1993. 21:2529–2530.13. Closs O, Harboe M, Axelsen NH, Bunch-Christensen K, magnusson M. The antigens of Mycobacterium bovis, strain BCG, studied by crossed immunoelectrophoresis: a reference system. Scand J Immunol. 1980. 12:249–263.14. Janicki BW, Chaparas SD, Daniel TM, Kubica GP, Wrigth GL, Yee GS. A reference system for antigens of Mycobacterium tuberculosis. Am Rev Respir Dis. 1971. 104:602–604.15. Young DB, Kaufmann SH, Hermans PW, Thole JE. Mycobacterial protein antigens: a compilation. Mol Microbiol. 1992. 6:133–145.16. Azuma I, Yamamura Y, Fukushi K. Fractionation of mycobacterial cell wall: isolation of arabinose mycolate and arabinogalactan from cell wall fraction of Mycobacterium tuberculosis strain Aoyama B. J Bacteriol. 1968. 96:1885–1887.17. Chaparas SD, Thor DE, Hedrick SR. Comparison of lymphocyte transformation, inhibition of macrophage migration and skin tests using dialyzable and nondialyzable tuberculin fractions from Mycobacterium bovis (BCG). J Immunol. 1971. 107:149–153.18. Yamamura Y, Onoue K, Azuma I. Biology of the mycobacterioses: chemical and immunological studies on peptides and polysaccharides from tubercle bacilli. Ann N Y Acad Sci. 1968. 154:88–97.19. Sorensen AL, Nagai S, Houen G, Andersen P, Andersen AB. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect Immun. 1995. 63:1710–1717.20. Chaparas SD, Vandiviere HM, Melvin I, Koch G, Becker C. Tuberculin test: variavility with the Mantoux procedure. Am Rev Respir Dis. 1985. 132:175–177.21. Sippola AA, Gillespie SL, Lewis JA, Daniel TM. Mycobacterium avium antigenuria in patients with AIDS and disseminated M. avium disease. J Infect Dis. 1993. 168:466–468.22. Choudhry V, Saxena RK. Detection of Mycobacterium tuberculosis antigens in urinary proteins of tuberculosis patients. Eur J Clin Microbiol Infect Dis. 2002. 21:1–5.23. Mazurek GH, Villarino ME. Guidelines for using the OuantiFERON-TB test for diagnosing latent Mycobacterium tuberculosis. MMWR Recomm Rep. 2003. 52:15–18.24. Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, et al. Specific detection of tuberculosis infection an interferon-gamma based assay using new antigens. Am J Respir Crit Care Med. 2004. 170:59–64.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of
Mycobacterium tuberculosis Early Secreted Antigenic Target 6 Recombinant Protein as a Diagnostic Marker in Skin Test - Prevention of tuberculosis and isolation of tuberculosis patients in health care facilities
- Expression of the 38 kDa Protein of Mycobacterium tuberculosis in M . bovis BCG and Use in the Serodiagnosis of Tuberculosis
- Purification of 30-kDa and 32 kDa protein antigens from mycobacterium tuberculosis and activation of human monocytes by lymphokines
- Isolation of perchloric acid soluble, heat stable, ethanol extractable protein from Mycobacterium tuberculosis
- Evaluation of