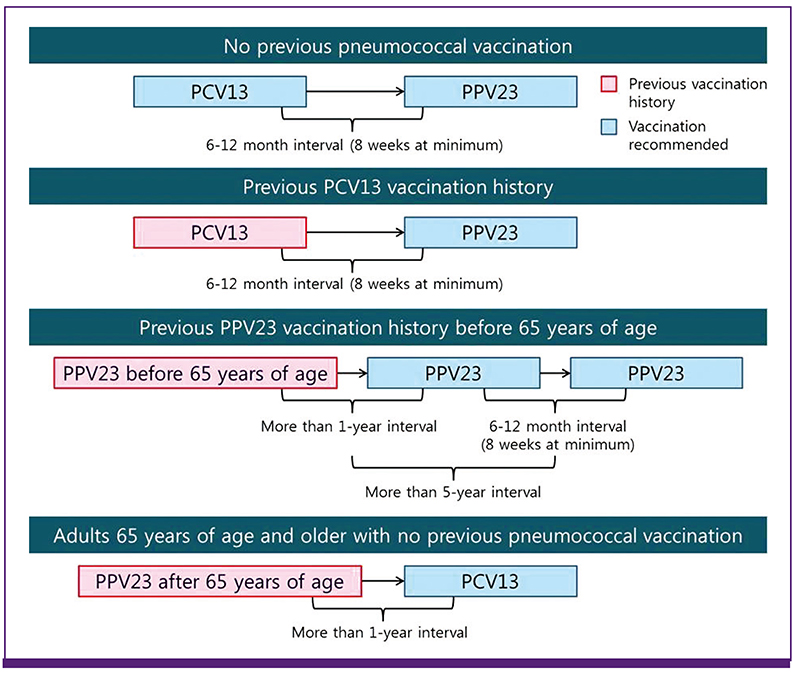
Revised Adult Immunization Guideline Recommended by the Korean Society of Infectious Diseases, 2014
- Affiliations
-
- 1Department of Internal Medicine, Korea University College of Medicine, Seoul, Korea. heejinmd@korea.ac.kr
- 2Department of Internal Medicine, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 3Department of Internal Medicine, Daegu Fatima Hospital, Daegu, Korea.
- 4Department of Obstetrics and Gynecology, Yonsei University College of Medicine, Seoul, Korea.
- 5Department of Internal Medicine, Ulsan University College of Medicine, Ulsan, Korea.
- 6Department of Pediatrics, Inha University College of Medicine, Incheon, Korea.
- 7Department of Internal Medicine, Inha University College of Medicine, Incheon, Korea.
- 8Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 9Department of Internal Medicine, Ewha Woman's University School of Medicine, Seoul, Korea.
- 10Department of Internal Medicine, Ajou University College of Medicine, Suwon, Korea.
- KMID: 1909527
- DOI: http://doi.org/10.3947/ic.2015.47.1.68
Abstract
- No abstract available.
Figure
Cited by 11 articles
-
Vaccination guideline for Immigrant in Korea by Korean Society of Infectious Diseases
Joon-Sup Yeom, Ki Tae Kwon, Jacob Lee, Yoo Bin Suh, Hae Suk Cheong, Hyun Hee Kwon, Hee Jin Cheong, ,
Infect Chemother. 2015;47(2):145-153. doi: 10.3947/ic.2015.47.2.145.Indirect Effects of Pneumococcal Conjugate Vaccines in National Immunization Programs for Children on Adult Pneumococcal Disease
Young Keun Kim, David LaFon, Moon H. Nahm
Infect Chemother. 2016;48(4):257-266. doi: 10.3947/ic.2016.48.4.257.Changes in Serotype of Streptococcus pneumoniae After the Introduction of the 13-Valent Pneumococcal Vaccine in a Homogenous Population on Jeju Island
Jeong Rae Yoo, Sang Taek Heo, Hyunjoo Oh, Suhyun Oh, Young Ree Kim, Keun Hwa Lee
Infect Chemother. 2019;51(1):67-72. doi: 10.3947/ic.2019.51.1.67.Vaccination in pregnancy
Min-Jeong Oh
J Korean Med Assoc. 2016;59(7):523-528. doi: 10.5124/jkma.2016.59.7.523.Updates of adult immunization in Korea
Hyun-Young Shin, Byung Wook Yoo
J Korean Med Assoc. 2020;63(2):128-134. doi: 10.5124/jkma.2020.63.2.128.Knowledge, Attitude and Practice on Maternal Immunization with Tetanus Toxoid, Reduced Diphtheria Toxoid, and Aellular Pertussis (Tdap) among Pregnant Women
Shin-Hye Lee, Bo-Kyeung Jin, Kyeung-Suk Baek, Yong-Sun Cho, Taek-Jin Lee
Pediatr Infect Vaccine. 2018;25(3):141-147. doi: 10.14776/piv.2018.25.e10.Knowledge, Attitude and Practice on Maternal Immunization with Tetanus Toxoid, Reduced Diphtheria Toxoid, and Aellular Pertussis (Tdap) among Pregnant Women
Shin-Hye Lee, Bo-Kyeung Jin, Kyeung-Suk Baek, Yong-Sun Cho, Taek-Jin Lee
Pediatr Infect Vaccine. 2018;25(3):141-147. doi: 10.14776/piv.2018.25.e10.Vaccination for Athletes: Evidence and Recommendations
Bumjo Oh, Young Koo Lee, Booyoon Cheung, Kyung Tai Lee, Chul-Won Ha
Korean J Sports Med. 2019;37(1):1-10. doi: 10.5763/kjsm.2019.37.1.1.Tetanus–diphtheria–acellular pertussis vaccination for adults: an update
Hyo-Jin Lee, Jung-Hyun Choi
Clin Exp Vaccine Res. 2017;6(1):22-30. doi: 10.7774/cevr.2017.6.1.22.Invasive Pneumococcal Disease Caused by Non-Vaccine Type Multidrug-Resistant
Streptococcus pneumoniae Transmitted by Close Contact in a Healthy Adult
Jeong Rae Yoo, Suhyun Oh, Jae-Geun Lee, Young Ree Kim, Keun Hwa Lee, Sang Taek Heo
Yonsei Med J. 2019;60(11):1103-1107. doi: 10.3349/ymj.2019.60.11.1103.Vaccination for Diabetic Patients: An Update
Joon-Sup Yeom
J Korean Diabetes. 2015;16(4):236-241. doi: 10.4093/jkd.2015.16.4.236.
Reference
-
1. Vila-Corcoles A, Ochoa-Gondar O. Preventing pneumococcal disease in the elderly: recent advances in vaccines and implications for clinical practice. Drugs Aging. 2013; 30:263–276.2. Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev. 2013; 1:CD000422.
Article3. Bonten M, Bolkenbaas M, Huijts S, Webber C, Gault S, Gruber W, Grobbee D. Community acquired pneumonia immunisation trial in adults (CAPITA). In : 9th International Symposium on Pneumococci and Pneumococcal Diseases; 2014 Mar 9-13; Hyderabad, India. ISPPD;2014. 3:p. 95.4. Jackson LA, Gurtman A, van Cleeff M, Jansen KU, Jayawardene D, Devlin C, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine compared to a 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults. Vaccine. 2003; 31:3577–3584.
Article5. Jackson LA, Gurtman A, Rice K, Pauksens K, Greenberg RN, Jones TR, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. 2013; 31:3585–3593.
Article6. Bryant K, Frenck R, Gurtman A, Rubino J, Treanor J, Thompson A, Jones TR, Jayawardene D, Baxter LM, Gruber WC, Emini EA, Scott DA, Schmöle-Thoma B. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults aged 18-49 years, naïve to 23-valent pneumococcal polysaccharide vaccine. 2013 April 28; Berlin, Germany. ESCMID.7. Ho YL, Brandão AP, de Cunto Brandileone MC, Lopes MH. Immunogenicity and safety of pneumococcal conjugate polysaccharide and free polysaccharide vaccines alone or combined in HIV-infected adults in Brazil. Vaccine. 2013; 31:4047–4053.
Article8. French N, Gordon SB, Mwalukomo T, White SA, Mwafulirwa G, Longwe H, Mwaiponya M, Zijlstra EE, Molyneux ME, Gilks CF. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N Engl J Med. 2010; 362:812–822.
Article9. Simonsen L, Taylor RJ, Schuck-Paim C, Lustig R, Haber M, Klugman KP. Effect of 13-valent pneumococcal conjugate vaccine on admissions to hospital 2 years after its introduction in the USA: a time series analysis. Lancet Respir Med. 2014; 2:387–394.
Article10. Harboe ZB, Dalby T, Weinberger DM, Benfield T, Mølbak K, Slotved HC, Suppli CH, Konradsen HB, Valentiner-Branth P. Impact of 13-valent pneumococcal conjugate vaccination in invasive pneumococcal disease incidence and mortality. Clin Infect Dis. 2014; 59:1066–1073.
Article11. Centers for Disease Control and Prevention (CDC). Licensure of 13-valent pneumococcal conjugate vaccine for adults aged 50 years and older. MMWR Morb Mortal Wkly Rep. 2012; 61:394–395.12. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine for adults with immunocompromising conditions: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2012; 61:816–819.13. Song JY, Choi JY, Lee JS, Bae IG, Kim YK, Sohn JW, Jo YM, Choi WS, Lee J, Park KH, Kim WJ, Cheong HJ. Clinical and economic burden of invasive pneumococcal disease in adults: a multicenter hospital-based study. BMC Infect Dis. 2013; 13:202.
Article14. Kim SH, Song JH, Chung DR, Thamlikitkul V, Yang Y, Wang H, Lu M, So TM, Hsueh PR, Yasin RM, Carlos CC, Pham HV, Lalitha MK, Shimono N, Perera J, Shibl AM, Baek JY, Kang CI, Ko KS, Peck KR. ANSORP Study Group. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012; 56:1418–1426.
Article15. Tomczyk S, Bennett NM, Stoecker C, Gierke R, Moore MR, Whitney CG, Hadler S, Pilishvili T. Centers for Disease Control and Prevention (CDC). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2014; 63:822–825.16. Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Sequential administration of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine in pneumococcal vaccine-naive adults 60-64 years of age. Vaccine. 2014; 32:2364–2374.
Article17. Jackson LA, Gurtman A, van Cleeff M, Frenck RW, Treanor J, Jansen KU, Scott DA, Emini EA, Gruber WC, Schmoele-Thoma B. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. 2013; 31:3594–3602.
Article18. Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women--Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013; 62:131–135.19. Leuridan E, Hens N, Peeters N, de Witte L, Van der Meeren O, Van Damme P. Effect of a prepregnancy pertussis booster dose on maternal antibody titers in young infants. Pediatr Infect Dis J. 2011; 30:608–610.
Article20. Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011; 204:334.e1–334.e5.
Article21. Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis. 2013; 56:539–544.
Article22. Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, Revzina NV, Kissin D, Lewis PW, Yue X, Haber P, Tokars JI, Vellozzi C, Broder KR. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol. 2012; 207:59.e1–59.e7.
Article23. Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011; 60:13–15.24. Centers for Disease Control and Prevention (CDC). Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) vaccine in adults aged 65 years and older - Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2012; 61:468–470.25. Akinsanya-Beysolow I. Advisory Committee on Immunization Practices (ACIP). ACIP Child/Adolescent Immunization Work Group. Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 0 through 18 years - United States, 2014. MMWR Morb Mortal Wkly Rep. 2014; 63:108–109.26. Public Health England. Pertussis (whooping cough) immunisation for pregnant women 2014. Accessed 12 June 2014. Available at: http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/WhoopingCough/ImmunisationForPregnantWomen/.27. Public Health Agency of Canada. Canadian immunization guide 2013. Accessed 12 June 2014. Available at: http://www.phac-aspc.gc.ca/publicat/cig-gci/p03-04-eng.php.28. Australian Government Deraprment of Health. The Australian immunisation handbook 10th Edition 2013. Accessed 12 June 2014. Available at: http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/handbook10-4-12.29. Korea Centers for Disease Control and Prevention (KCDC). Disease web statistics system 2014. Accessed 12 June 2014. Available at: http://is.cdc.go.kr/nstat/jsp/observation/stat/tot/STATTOT0402List.jsp.30. Kwon HJ, Yum SK, Choi UY, Lee SY, Kim JH, Kang JH. Infant pertussis and household transmission in Korea. J Korean Med Sci. 2012; 27:1547–1551.
Article31. Schmader KE, Levin MJ, Gnann JW Jr, McNeil SA, Vesikari T, Betts RF, Keay S, Stek JE, Bundick ND, Su SC, Zhao Y, Li X, Chan IS, Annunziato PW, Parrino J. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50-59 years. Clin Infect Dis. 2012; 54:922–928.
Article32. Centers for Disease Control and Prevention (CDC). Update on herpes zoster vaccine: licensure for persons aged 50 through 59 years. MMWR Morb Mortal Wkly Rep. 2011; 60:1528.33. Giuliano AR, Palefsky JM, Goldstone S, Moreira ED Jr, Penny ME, Aranda C, Vardas E, Moi H, Jessen H, Hillman R, Chang YH, Ferris D, Rouleau D, Bryan J, Marshall JB, Vuocolo S, Barr E, Radley D, Haupt RM, Guris D. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011; 364:401–411.
Article34. Palefsky JM, Giuliano AR, Goldstone S, Moreira ED Jr, Aranda C, Jessen H, Hillman R, Ferris D, Coutlee F, Stoler MH, Marshall JB, Radley D, Vuocolo S, Haupt RM, Guris D, Garner EI. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011; 365:1576–1585.
Article35. Centers for Disease Control and Prevention (CDC). Recommendations on the use of quadrivalent human papillomavirus vaccine in males--Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011; 60:1705–1708.36. Anonymous. Global advisory committee on vaccine safety, 11-12 December 2013. Wkly Epidemiol Rec. 2014; 89:53–60.37. Anonymous. Global advisory committee on vaccine safety, 12-13 June 2013. Wkly Epidemiol Rec. 2013; 88:301–312.38. Ministry of Food and Drug Safety. Media press release. Accessed 3 March 2014. Available at: http://www.mfds.go.kr/index.do?mid=676&pageNo=1&seq=23066&cmd=v.39. U.S. Food and Drug Administrarion. Study Reports Aluminum in Vaccines Poses Extremely Low Risk to Infants. Accessed 5 January 2012. http://www.fda.gov/biologicsbloodvaccines/scienceresearch/ucm284520.htm.40. World Health Organization (WHO). Global Advisory Committee on Vaccine Safety, report of meeting held 6-7 June 2012. Accessed 5 January 2012. Available at: http://www.who.int/vaccine_safety/committee/reports/Jun_2012/en/.41. Harris JW. Influenza occurring in pregnant women: a statistical study of thirteen hundred and fifty cases. J Am Med Assoc. 1919; 72:978–980.42. Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol. 1959; 78:1172–1175.
Article43. Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, Fonseca VP, Ritger KA, Kuhles DJ, Eggers P, Bruce H, Davidson HA, Lutterloh E, Harris ML, Burke C, Cocoros N, Finelli L, MacFarlane KF, Shu B, Olsen SJ. Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet. 2009; 374:451–458.
Article44. Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, Moore Z, Wiedeman C, Anand M, Tabony L, Nielsen CF, Waller K, Page S, Thompson JM, Avery C, Springs CB, Jones T, Williams JL, Newsome K, Finelli L, Jamieson DJ. Pandemic H1N1 Influenza in Pregnancy Working Group. Pandemic 2009 Influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010; 303:1517–1525.
Article45. Mosby LG, Rasmussen SA, Jamieson DJ. 2009 pandemic influenza A (H1N1) in pregnancy: a systematic review of the literature. Am J Obstet Gynecol. 2011; 205:10–18.
Article46. Anonymous. Vaccines against influenza WHO position paper - November 2012. Wkly Epidemiol Rec. 2012; 87:461–476.47. Kim BN, Kwak YG, Moon CS, Kim YS, Kim ES, Lee KS, Lee CS, Hur JA. Pandemic influenza (H1N1 2009) among pregnant Korean women. Infect Chemother. 2011; 43:55–59.
Article48. An JH, Kim HN, Choi OJ, Kim GS, Kim UJ, Jang MO, Kang SJ, Park KH, Jung SI, Kwon YS, Jang HC. Was 2009 pandemic influenza A (H1N1) mild among pregnant Korean women? Chonnam Med J. 2013; 49:96–99.
Article49. Thompson MG, Li DK, Shifflett P, Sokolow LZ, Ferber JR, Kurosky S, Bozeman S, Reynolds SB, Odouli R, Henninger ML, Kauffman TL, Avalos LA, Ball S, Williams JL, Irving SA, Shay DK, Naleway AL. Pregnancy and Influenza Project Workgroup. Effectiveness of seasonal trivalent influenza vaccine for preventing influenza virus illness among pregnant women: a population-based case-control study during the 2010-2011 and 2011-2012 influenza seasons. Clin Infect Dis. 2014; 58:449–457.
Article50. Legge A, Dodds L, MacDonald NE, Scott J, McNeil S. Rates and determinants of seasonal influenza vaccination in pregnancy and association with neonatal outcomes. CMAJ. 2014; 186:E157–E164.
Article51. Nordin JD, Kharbanda EO, Vazquez Benitez G, Lipkind H, Vellozzi C, Destefano F. Vaccine Safety Datalink. Maternal influenza vaccine and risks for preterm or small for gestational age birth. J Pediatr. 2014; 164:1051–1057. e2.
Article52. Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008; 359:1555–1564.
Article53. Kim IS, Seo YB, Hong KW, Noh JY, Choi WS, Song JY, Cho GJ, Oh MJ, Kim HJ, Hong SC, Sohn JW, Cheong HJ, Kim WJ. Perception on influenza vaccination in Korean women of childbearing age. Clin Exp Vaccine Res. 2012; 1:88–94.
Article54. Kim MJ, Lee SY, Lee KS, Kim A, Son D, Chung MH, Park SG, Park JH, Lee BI, Lee JS. Influenza vaccine coverage rate and related factors on pregnant women. Infect Chemother. 2009; 41:349–354.
Article55. Lee YK, Kwon Y, Kim DW, Song KM, Cho H, Kim CH, Go UY, Bae GR, Lee JK. 2009-2010 novel influenza A (H1N1) vaccination coverage in the Republic of Korea. Am J Infect Control. 2012; 40:481–483.
Article56. Noh JY, Kim WJ. Influenza vaccines: unmet needs and recent developments. Infect Chemother. 2013; 45:375–386.
Article57. Korea Centers for Disease Control and Prevention (KCDC). Korean influenza surveillance report, 2012-2013. Accessed 15 March 2014. Available at: http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0037-MNU1380&q_type=&year=2013&cid=21953&pageNum=.58. Korea Centers for Disease Control and Prevention (KCDC). Korean influenza surveillance report, 2011-2012. Accessed 15 March 2014. Available at: http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0037-MNU1380&q_type=&year=2012&cid=19407&pageNum=.59. Korea Centers for Disease Control and Prevention (KCDC). Korean influenza surveillance report, 2010-2011. Accessed 15 March 2014. Available at: http://www.cdc.go.kr/CDC/info/CdcKrInfo0301.jsp?menuIds=HOME001-MNU1132-MNU1138-MNU0037-MNU1380&q_type=&year=2011&cid=12720&pageNum=.60. Eurosurveillance editorial team. WHO recommendations on the composition of the 2013/14 influenza virus vaccines in the northern hemisphere. Euro Surveill. 2013; 18:pii: 20411.61. Kieninger D, Sheldon E, Lin WY, Yu CJ, Bayas JM, Gabor JJ, Esen M, Fernandez Roure JL, Narejos Perez S, Alvarez Sanchez C, Feng Y, Claeys C, Peeters M, Innis BL, Jain V. Immunogenicity, reactogenicity and safety of an inactivated quadrivalent influenza vaccine candidate versus inactivated trivalent influenza vaccine: a phase III, randomized trial in adults aged ≥18 years. BMC Infect Dis. 2013; 13:343.62. Pépin S, Donazzolo Y, Jambrecina A, Salamand C, Saville M. Safety and immunogenicity of a quadrivalent inactivated influenza vaccine in adults. Vaccine. 2013; 31:5572–5578.
Article63. Jain VK, Rivera L, Zaman K, Espos RA Jr, Sirivichayakul C, Quiambao BP, Rivera-Medina DM, Kerdpanich P, Ceyhan M, Dinleyici EC, Cravioto A, Yunus M, Chanthavanich P, Limkittikul K, Kurugol Z, Alhan E, Caplanusi A, Durviaux S, Boutet P, Ofori-Anyinam O, Chandrasekaran V, Dbaibo G, Innis BL. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med. 2013; 369:2481–2491.
Article64. Korea Centers for Disease Control and Prevention (KCDC). Disease web statistics system. Accessed 9 December 2014. Available at: http://is.cdc.go.kr/nstat/index.jsp.65. Kim SA, Kim DW, Dong BQ, Kim JS, Anh DD, Kilgore PE. An expanded age range for meningococcal meningitis: molecular diagnostic evidence from population-based surveillance in Asia. BMC Infect Dis. 2012; 12:310.
Article66. Cho HK, Lee H, Kang JH, Kim KN, Kim DS, Kim YK, Kim JS, Kim JH, Kim CH, Kim HM, Park SE, Oh SH, Chung EH, Cha SH, Choi YY, Hur JK, Hong YJ, Lee HJ, Kim KH. The causative organisms of bacterial meningitis in Korean children in 1996-2005. J Korean Med Sci. 2010; 25:895–899.
Article67. Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE. Centers for Disease Control and Prevention (CDC). Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013; 62:1–28.68. Korea Centers for Diseases Control and Prevention (KCDC). Epidemiology and prevention of vaccine-preventable disease. 4th ed. Osong, Korea: KCDC;2013.69. Korea Centers for Diseases Control and Prevention (KCDC). National immunization program. Accessed 9 December 2014. Available at: https://nip.cdc.go.kr/irgd/index.html.70. Anonymous. Meeting of the Strategic Advisory Group of Experts on immunization, April 2013 - conclusions and recommendations. Wkly Epidemiol Rec. 2013; 88:201–206.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recommended immunization schedule for adults in Korea, by the Korean Society of Infectious Diseases, 2012
- Recommended immunization schedule for children and adolescents in Korea, the Korean Pediatric Society, 2013 (The Committee on Infectious Diseases, the Korean Pediatric Society)
- Adult Immunization in Patients with Diabetes Mellitus: Current Immunization Status and Recommended Schedule in Korea
- Revised Guideline of Immunization
- Active Immunization