Korean J Hematol.
2012 Dec;47(4):260-266. 10.5045/kjh.2012.47.4.260.
Simplified flow cytometric immunophenotyping panel for multiple myeloma, CD56/CD19/CD138(CD38)/CD45, to differentiate neoplastic myeloma cells from reactive plasma cells
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. cjpark@amc.seoul.kr
- 2Department of Laboratory Medicine, Ajou University School of Medicine, Suwon, Korea.
- 3Department of Internal Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea.
- KMID: 1832104
- DOI: http://doi.org/10.5045/kjh.2012.47.4.260
Abstract
- BACKGROUND
Flow cytometric immunophenotyping has been used to identify neoplastic plasma cell populations in patients with multiple myeloma (MM). Previous reports have described the use of several antigens, including CD38, CD138, CD56, CD117, CD52, CD19 and CD45, to distinguish distinct populations of plasma cells. The aim of this study was to evaluate a simplified immunophenotyping panel for MM analysis.
METHODS
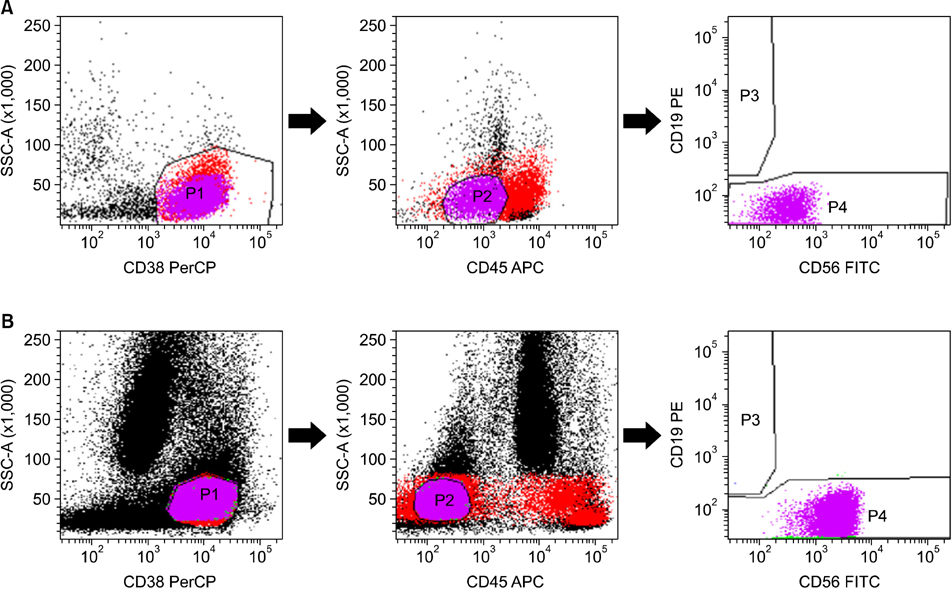
A total of 70 patients were enrolled in the study, 62 of which were newly diagnosed with MM (untreated), whereas the remaining 8 were undergoing bone marrow assessment as part of follow-up after treatment (treated). Treated cases included 3 patients with relapse and 5 patients with persistence of MM. Multiparametric flow cytometric immunophenotyping was performed using monoclonal antibodies against CD56, CD19, CD138 (CD38), and CD45.
RESULTS
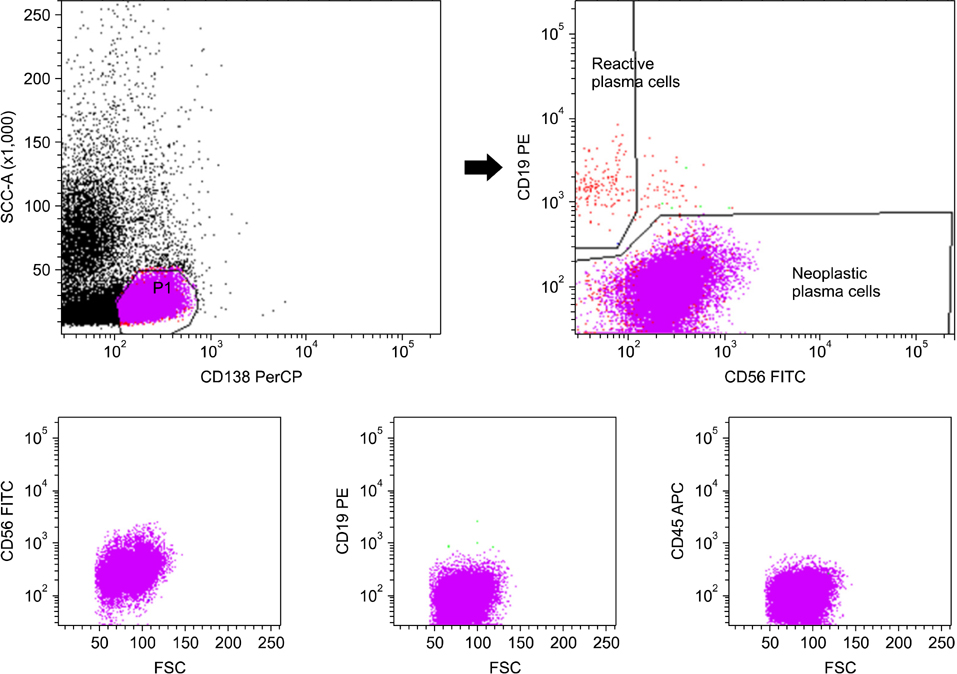
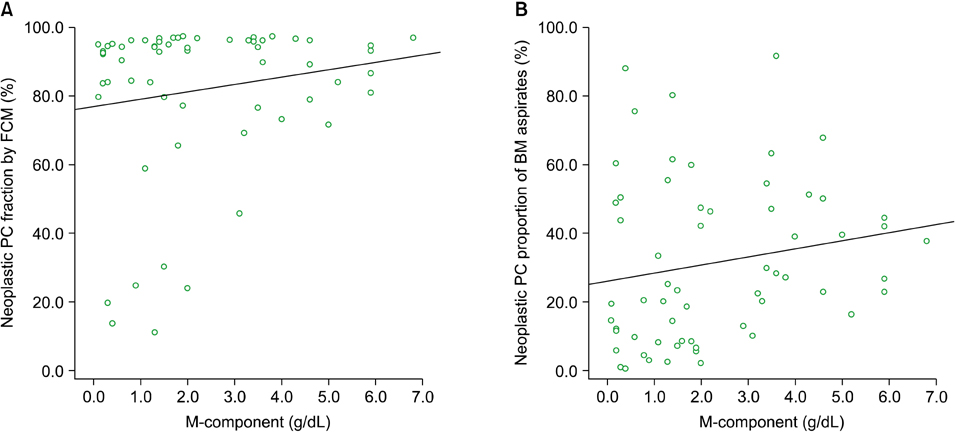
In differential counts, plasma cells in bone marrow (BM) accounted for 3.6-93.2% of the total nucleated cell count. The positive expression rates of CD56, CD19, CD138, and CD45 in neoplastic myeloma cells were 83.9%, 0%, 98.4%, and 37.1%, respectively, among the 62 untreated cases, and 75.0%, 0%, 87.5%, and 37.5%, respectively, among the 8 treated cases. CD19 expression of neoplastic plasma cells was negative in both untreated and treated cases.
CONCLUSION
The simplified immunophenotyping panel, CD56/CD19/CD138(CD38)/CD45, is useful for distinguishing neoplastic myeloma cells from reactive plasma cells in clinical practice. In addition, CD19 represents the most valuable antigen for identifying neoplastic myeloma cells in patients with MM.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
반응성 형질세포와 악성 형질세포 감별을 위한 형질세포 선별튜브의 유용성 평가
Hae In Bang, Tae Youn Choi
Lab Med Online. 2020;10(4):314-320. doi: 10.47429/lmo.2020.10.4.314.Phenotypic consensus markers for plasma cell myeloma
Mina Hur
Korean J Hematol. 2012;47(4):239-240. doi: 10.5045/kjh.2012.47.4.239.
Reference
-
1. Lin P. Plasma cell myeloma. Hematol Oncol Clin North Am. 2009. 23:709–727.
Article2. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011. 117:4701–4705.
Article3. Harada H, Kawano MM, Huang N, et al. Phenotypic difference of normal plasma cells from mature myeloma cells. Blood. 1993. 81:2658–2663.
Article4. Van Camp B, Durie BG, Spier C, et al. Plasma cells in multiple myeloma express a natural killer cell-associated antigen: CD56 (NKH-1; Leu-19). Blood. 1990. 76:377–382.
Article5. Wijdenes J, Vooijs WC, Clément C, et al. A plasmocyte selective monoclonal antibody (B-B4) recognizes syndecan-1. Br J Haematol. 1996. 94:318–323.
Article6. Ocqueteau M, Orfao A, Almeida J, et al. Immunophenotypic characterization of plasma cells from monoclonal gammopathy of undetermined significance patients. Implications for the differential diagnosis between MGUS and multiple myeloma. Am J Pathol. 1998. 152:1655–1665.7. Mahmoud MS, Fujii R, Ishikawa H, Kawano MM. Enforced CD19 expression leads to growth inhibition and reduced tumorigenicity. Blood. 1999. 94:3551–3558.
Article8. Guikema JE, Hovenga S, Vellenga E, et al. CD27 is heterogeneously expressed in multiple myeloma: low CD27 expression in patients with high-risk disease. Br J Haematol. 2003. 121:36–43.
Article9. Robillard N, Avet-Loiseau H, Garand R, et al. CD20 is associated with a small mature plasma cell morphology and t(11;14) in multiple myeloma. Blood. 2003. 102:1070–1071.
Article10. Kovarova L, Buresova I, Buchler T, et al. Phenotype of plasma cells in multiple myeloma and monoclonal gammopathy of undetermined significance. Neoplasma. 2009. 56:526–532.
Article11. Sezer O, Heider U, Zavrski I, Possinger K. Differentiation of monoclonal gammopathy of undetermined significance and multiple myeloma using flow cytometric characteristics of plasma cells. Haematologica. 2001. 86:837–843.12. Rawstron AC, Orfao A, Beksac M, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008. 93:431–438.
Article13. Jaffe ES, Harris NL, Stein H, Vardiman JW, editors. WHO Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. 2001. Volume 3. Lyon, France: IARC Press.14. Sandoval-Montes C, Santos-Argumedo L. CD38 is expressed selectively during the activation of a subset of mature T cells with reduced proliferation but improved potential to produce cytokines. J Leukoc Biol. 2005. 77:513–521.
Article15. Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004. 121:482–488.
Article16. Reid S, Yang S, Brown R, et al. Characterisation and relevance of CD138-negative plasma cells in plasma cell myeloma. Int J Lab Hematol. 2010. 32:e190–e196.
Article17. Rawstron AC. Immunophenotyping of plasma cells. Curr Protoc Cytom. 2006. Chapter 6:Unit6.23.
Article18. Sahara N, Takeshita A, Shigeno K, et al. Clinicopathological and prognostic characteristics of CD56-negative multiple myeloma. Br J Haematol. 2002. 117:882–885.
Article19. Kumar S, Rajkumar SV, Kimlinger T, Greipp PR, Witzig TE. CD45 expression by bone marrow plasma cells in multiple myeloma: clinical and biological correlations. Leukemia. 2005. 19:1466–1470.
Article20. Pellat-Deceunynck C, Bataille R. Normal and malignant human plasma cells: proliferation, differentiation, and expansions in relation to CD45 expression. Blood Cells Mol Dis. 2004. 32:293–301.
Article21. Raja KR, Kovarova L, Hajek R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br J Haematol. 2010. 149:334–351.
Article22. Chang CC, Schur BC, Kampalath B, Lindholm P, Becker CG, Vesole DH. A novel multiparametric approach for analysis of cytoplasmic immunoglobulin light chains by flow cytometry. Mod Pathol. 2001. 14:1015–1021.
Article23. Barlogie B, Alexanian R, Pershouse M, Smallwood L, Smith L. Cytoplasmic immunoglobulin content in multiple myeloma. J Clin Invest. 1985. 76:765–769.
Article24. Slaper-Cortenbach IC, Admiraal LG, Kerr JM, van Leeuwen EF, von dem Borne AE, Tetteroo PA. Flow-cytometric detection of terminal deoxynucleotidyl transferase and other intracellular antigens in combination with membrane antigens in acute lymphatic leukemias. Blood. 1988. 72:1639–1644.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Utility of Plasma Cell Screening Tube Kit to Differentiate Neoplastic Plasma Cells from Reactive Plasma Cells
- Immunophenotypic Characterization and Quantification of Neoplastic Bone Marrow Plasma Cells by Multiparametric Flow Cytometry and Its Clinical Significance in Korean Myeloma Patients
- The Increased Expression and Diagnostic Usefulness of CD56 Antigen in Paraffin Embedded Plasma Cell Neoplasm
- A Case of Anaplastic Myeloma: Role of Immunophenotyping in Initial Diagnosis
- Auer rod-like crystal inclusions in plasma cells of multiple myeloma