Korean Circ J.
2009 Mar;39(3):105-110. 10.4070/kcj.2009.39.3.105.
Beneficial and Adverse Effects of Bosentan Treatment in Korean Patients With Pulmonary Artery Hypertension
- Affiliations
-
- 1Division of Cardiology, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea. dwsohn@snu.ac.kr
- 2Division of Rheumatology, Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea.
- 4Division of Cardiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 5Division of Pediatric Cardiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 6Department of Pulmonology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 7Department of Pulmonology & Clinical Care Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 8Division of Pediatric Cardiac Surgery and Cardiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea.
- 9Division of Cardiology, Department of Internal Medicine, St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea.
- 10Division of Cardiology, Yonsei University College of Medicine, Seoul, Korea.
- 11Division of Pediatric Cardiology, Yonsei University College of Medicine, Seoul, Korea.
- 12Department of Internal Medicine, The Hospital for Rheumatic Diseases, Hanyang University College of Medicine, Seoul, Korea.
- 13Division of Cardiology, Hanyang University College of Medicine, Seoul, Korea.
- KMID: 1825967
- DOI: http://doi.org/10.4070/kcj.2009.39.3.105
Abstract
-
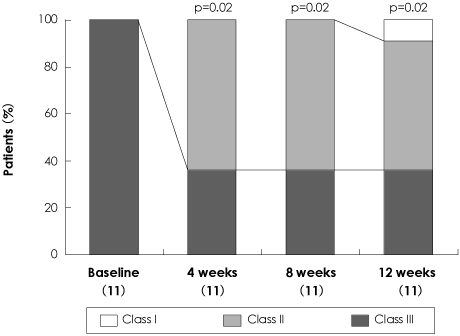
BACKGROUND AND OBJECTIVES: The purpose of this study was to investigate 1) the beneficial effect of bosentan treatment (125 mg twice daily) on exercise capacity and echocardiographic variables and 2) the profiles and frequency of adverse events in Korean patients with World Health Organization (WHO) class III or IV pulmonary artery hypertension (PAH).
SUBJECTS AND METHODS
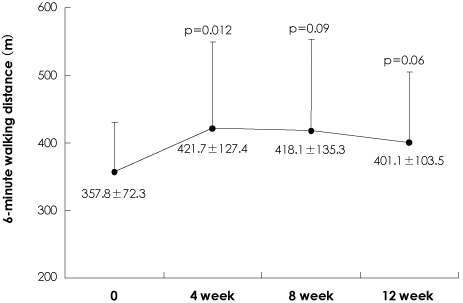
Twelve patients who received bosentan treatment were investigated in an open label manner. One patient was excluded in the final analyses due to a prohibited concomitant medication. A 6-minute walk test and echocardiography were performed at baseline and after 12 weeks of treatment.
RESULTS
The administration of bosentan for 12 weeks resulted in a significant improvement in exercise capacity (measured with the 6-minute walking distance), WHO functional capacity, and in echocardiographic variables. Bosentan treatment was associated with a decrease in the maximal tricuspid regurgitation jet velocity {from 4.7 m/sec (95% confidence interval, 3.89-5.45) at baseline to 4.4 m/sec (95% confidence interval, 3.61-5.1) at 12 weeks, p=0.03} and systolic pulmonary arterial pressure {from 105 mmHg (95% confidence interval, 74.4-135.6) at baseline to 93 mmHg (95% confidence interval, 66.3-120.1) at 12 weeks, p=0.04}. Treatment with bosentan at a dose of 125 mg twice a day was not associated with life-threatening side effects, although a higher incidence of elevated liver enzymes compared to previous studies was noted.
CONCLUSION
Bosentan at a dose of 125 mg twice daily is considered a clinically optimal, safe dose and can be used as a valuable treatment option in Korean PAH patients with WHO functional capacity III or IV, though close monitoring of liver function is required.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Functional Class and Targeted Therapy Are Related to the Survival in Patients with Pulmonary Arterial Hypertension
Yae Min Park, Wook-Jin Chung, Deok Young Choi, Han Joo Baek, Sung Hwan Jung, In Suck Choi, Eak Kyun Shin
Yonsei Med J. 2014;55(6):1526-1532. doi: 10.3349/ymj.2014.55.6.1526.
Reference
-
1. Rubin LJ. Primary pulmonary hypertension. N Engl J Med. 1997. 336:111–117.2. D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension: results from a national prospective registry. Ann Intern Med. 1991. 115:343–349.3. Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988. 332:411–415.4. McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006. 114:1417–1431.5. Giaid A, Yanagisawa M, Langleben D, et al. Expression of endothelin-1 in the lungs of patients with pulmonary hypertension. N Engl J Med. 1993. 328:1732–1739.6. Rubens C, Ewert R, Halank M, et al. Big endthelin-1 and endothelin-1 plasma levels are correlated with severity of primary pulmonary hypertension. Chest. 2001. 120:1562–1569.7. Channnick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomized placebo-controlled study. Lancet. 2001. 358:1119–1123.8. Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002. 346:896–903.9. McLaughlin VV, Sitbon O, Badesch DB, et al. Survival with first-line bosentan in patients with primary pulmonary hypertension. Eur Respir J. 2005. 25:244–249.10. Galie N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. Eur Heart J. 2004. 25:2243–2278.11. Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med. 2004. 351:1425–1436.12. Galie N, Seeger W, Naeije R, Simonneau G, Rubin LJ. Comparative analysis of clinical trials and evidence-based treatment algorithm in pulmonary arterial hypertension. J Am Coll Cardiol. 2004. 43:12 Suppl S. 81S–88S.13. Kim HK, Kim YJ, Park JS, et al. Determinants of the severity of functional tricuspid regurgitation. Am J Cardiol. 2006. 98:236–242.14. Sagie A, Schwammenthal E, Padial LR, Vazquez de Prada JA, Weyman AE, Levine RA. Determinants of functional tricuspid regurgitation in incomplete tricuspid valve closure: Doppler color flow study of 109 patients. J Am Coll Cardiol. 1994. 24:446–453.15. Kircher BJ, Himelman RB, Schiller NB. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am J Cardiol. 1990. 66:493–496.16. Hinderliter AL, Willis PW 4th, Barst RJ, et al. Effect of long-term infusion of prostacyclin (epoprostenol) on echocardiographic measures of right ventricular structure and function in primary pulmonary hypertension. Circulation. 1997. 95:1479–1486.17. Hinderliter AL, Willis PW 4th, Long W, et al. Frequency and prognostic significance of pericardial effusion in primary pulmonary hypertension. Am J Cardiol. 1999. 84:481–484.18. Badesch DB, Abman SH, Simonneau G, Rubin LJ, McLaughlin VV. Medical therapy for pulmonary arterial hypertension: updated ACCP evidence-based clinical practice guidelines. Chest. 2007. 131:1917–1928.19. Sitbon O, McLaughlin VV, Badesch DB, et al. Survival in patients with class III idiopathic pulmonary arterial hypertension treated with first line oral bosentan compared with an historical cohort of patients started on intravenous epoprostenol. Thorax. 2005. 60:1025–1030.20. Sasayama S, Kunieda T, Tomoike H, et al. Effects of the endothelin receptor antagonist bosentan on hemodynamics, symptoms and functional capacity in Japanese patients with severe pulmonary hypertension. Circ J. 2005. 69:131–137.21. Sasayama S, Satoh T, Izumi T, Yoshida S, Kyotani S, Tahara N. Long-term trial of bosentan monotherapy for pulmonary arterial hypertension in Japanese patients. Curr Med Res Opin. 2007. 23:395–400.22. Bossone E, Duong-Wagner TH, Paciocco G, et al. Echocardiographic features of primary pulmonary hypertension. J Am Soc Echocardiogr. 1999. 12:655–662.23. Channick RN, Simonneau G, Sitbon O, et al. Effects of the dual endothelin-receptor antagonist bosentan in patients with pulmonary hypertension: a randomised placebo-controlled study. Lancet. 2001. 358:1119–1123.24. Galié N, Hinderliter AL, Torbicki A, et al. Effects of the oral endothelin-receptor antagonist bosentan on echocardiographic and Doppler measures in patients with pulmonary arterial hypertension. J Am Coll Cardiol. 2003. 41:1380–1386.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Bosentan Treatment on Angiotensin Converting Enzyme in Monocrotaline Induced Pulmonary Hypertension Rats
- Meta-analysis of randomized controlled trials of bosentan for treatment of pulmonary arterial hypertension
- Transient Use of Oral Bosentan Can Be an Additional Option to Reduce Pulmonary Arterial Hypertension in a Patient with Severe Pulmonary Arterial Hypertension Associated with Atrial Septal Defect
- Gene Expression of Endothelin-1 and Endothelin Receptor A on Monocrotaline-Induced Pulmonary Hypertension in Rats After Bosentan Treatment
- Effect of endothelin receptor blockade on monocrotaline-induced pulmonary hypertension in rats